
674
Thorax 1997;52:674–679
Original articles
Effect of long term oxygen therapy on survival
in patients with chronic obstructive pulmonary
disease with moderate hypoxaemia
Dorota Go
´
recka, Katarzyna Gorzelak, Paweł S
´
liwin
´
ski, Mirosław Tobiasz, Jan Zielin
´
ski
Abstract Patients with COPD are usually given LTOT
in the advanced stage of the disease and longBackground – To date only two controlled
studies have been published on the effects term survival in such patients, despite oxygen
treatment, remains poor.
1–4
It has been sug-of domiciliary oxygen treatment on sur-
vival in patients with chronic obstructive gested that LTOT should be prescribed earlier
in the natural history of the disease,
56
and inpulmonary disease (COPD) with ad-
vanced respiratory failure. The survival some countries oxygen is also prescribed to
patients with moderate hypoxaemia (Pa
2
in such patients despite oxygen treatment
remains poor. The prescription of long 7.4–8.7 kPa (56–65 mmHg)).
78
However, no
controlled studies have been reported to showterm oxygen therapy (LTOT) in less severe
disease remains controversial. The aim of that the implementation of LTOT in this group
of patients also prolongs life. The aim of ourthis study was to evaluate the rationale for
prescribing oxygen to patients with COPD study was therefore to evaluate the rationale of
prescribing oxygen in patients with COPD withwith moderate hypoxaemia.
Methods – One hundred and thirty five moderate hypoxaemia.
patients with COPD, with Pa
O
2
7.4–8.7 kPa
(56–65 mmHg) and advanced airflow lim-
itation (mean (SD) forced expiratory vol-
Methods
ume in one second (FEV
1
) 0.83 (0.28) l),
One hundred and thirty five consecutive
were randomly allocated to a control (n=
patients with COPD referred to nine regional
67) and LTOT (n=68) group. The patients
LTOT centres in Poland with moderate hyp-
were followed every three months for at
oxaemia (Pa
2
7.4–8.7 kPa (56–65 mmHg))
least three years or until death.
entered the study in the years 1987–92 and
Results –The cumulative survival rate was
were followed up to the end of 1994. The
88% at one year, 77% at two years, and 66%
organisation of domiciliary oxygen therapy in
at three years. No significant differences
Poland and qualification procedures have been
were found in survival rates between
described previously.
910
patients treated with LTOT and controls,
We included patients with COPD as a single
nor did longer oxygen use (over 15 hours
diagnosis, aged between 40 and 80 years, with
per day) improve survival. Younger age,
airway limitation defined by FEV
1
/VC post
better spirometric values, and higher body
bronchodilator of <70%. Patients with serious
mass index predicted better survival.
disease of organs other than the lungs that
Conclusions – Domiciliary oxygen treat-
might influence survival were excluded from
ment does not prolong survival in patients
the study. Baseline studies included a complete
with COPD with moderate hypoxaemia.
history, physical examination, and basic
Airway limitation seems to determine sur-
laboratory tests. Spirometric measurements
vival in this group of patients.
and blood gas tensions were measured twice,
Department of
(Thorax 1997;52:674–679)
at least three weeks apart, in all patients, along
Respiratory Medicine,
Institute of
with a chest radiograph and ECG before en-
Tuberculosis and Lung
Keywords: chronic obstructive pulmonary disease, mod-
tering the study. Patients were randomly al-
Diseases, 01-138
erate hypoxaemia, long term oxygen therapy, survival.
located to receive either conventional treatment
Warsaw, Poland
DGo
´
recka
(controls) or conventional treatment plus oxy-
K Gorzelak
Long term oxygen therapy (LTOT) is generally gen (LTOT). Randomisation schedules were
PS
´
liwin
´
ski
accepted as a therapeutic measure in patients developed centrally. Treatment assignments
M Tobiasz
J Zielin
´
ski
with chronic respiratory failure. Although were computer generated by random numbers,
with an equal number of patients in the controlLTOT is prescribed in various lung diseases
Correspondence to:
Dr D Go
´
recka.
leading to chronic hypoxia, its beneficial and treatment groups. Usual treatment con-
sisted of bronchodilators (theophylline, b
2
Received 9 July 1996
effects have only been evaluated in patients
Returned to authors
with chronic obstructive pulmonary disease agonists, and anticholinergic drugs). Anti-
21 October 1996
Revised version received
(COPD) and severe hypoxaemia (Pa
2
biotics, diuretics, and corticosteroids were pre-
29 January 1997
<8.0 kPa (60 mmHg)) in whom a substantial scribed at the discretion of the physician.
Accepted for publication
30 January 1997
improvement in survival has been shown.
12
Prolonged use of corticosteroids was defined
Effect of LTOT on survival in patients with COPD 675
Table 2 Causes of death in control and LTOT groupsTable 1 Mean (SD) clinical characteristics of 135 patients with COPD at entry to the
study
Causes of death Control group LTOT group
(n=32) (n=38)
Variable Total Control group LTOT group
(n=135) (n=67) (n=68)
COPD 22 21
Myocardial infarction 3 1
Age (years) 61.2 (8.5) 62.4 (8.2) 60.1 (8.8)
Sudden death at home — 6
M/F 103/32 52/15 51/17
Death during sleep 1 2
BMI (kg/m
2
) 23.6 (6.0) 23.3 (4.0) 23.8 (5.1)
Lung cancer 1 2
Pa
2
(kPa/mmHg) 8.0 (0.4)/60.4 (2.8) 8.2 (0.4)/61.3 (2.7) 7.9 (0.4)/59.5 (2.7)∗
Other neoplasm 1 2
Pa
2
(kPa/mmHg) 5.9 (0.9)/44.1 (6.7) 5.7 (0.9)/42.8 (6.6) 6.0 (0.9)/45.3 (6.7)
Pulmonary embolism 1 1
VC (l) 1.95 (0.59) 1.98 (0.54) 1.94 (0.64)
Gastric haemorrhage 1 1
VC (% pred) 48.9 (11.9) 50.0 (11.6) 47.7 (12.2)
Suicide — 1
FEV
1
(l) 0.83 (0.28) 0.81 (0.29) 0.85 (0.28)
Pneumothorax — 1
FEV
1
(% pred) 29.8 (9.8) 29.8 (10.3) 29.7 (9.4)
Cerebral haemorrhage 1 —
FEV
1
/VC (%) 42.9 (12.9) 40.8 (12.1) 45.1 (13.4)
Car accident 1 —
Haematocrit (%) 47.2 (5.5) 46.4 (5.3) 47.9 (5.7)
Observation time (months) 40.9 (19.9) 38.9 (19.7) 42.8 (20.1)
Steroids (no. of pts) 39 20 19
O
2
use (hours) 13.5 (4.4)
BMI=body mass index; Pa
2
=arterial partial oxygen tension; Pa
2
=arterial partial carbon
40–79). On average the patients were observed
dioxide tension; VC=vital capacity; FEV
1
=forced expiratory volume in one second.
for 40.9 months (range 2–85). Mean values of
∗ p<0.05 control versus LTOT group.
body mass index (BMI), spirometric values,
blood gas tensions, and haematocrit at entry
to the study are shown in table 1. All patients
as lasting more than six months. Patients on
suffered from severe airflow limitation with
LTOT received oxygen from an oxygen con-
mean (SD) forced expiratory volume in one
centrator at a flow rate adjusted to raise resting
second (FEV
1
) 0.83 (0.28) l.
Pa
2
above 8.7 kPa (65 mmHg). The pre-
The control and treatment groups were well
scribed oxygen breathing time was at least 17
matched in all measured variables. The only
hours per 24 hours. The compliance with the
significant difference at entry to the study was
treatment was checked by reading the oxygen
the value of Pa
2
(table 1). To check for the
meter built into the oxygen concentrator.
influence of Pa
2
on survival the Cox’s re-
Patients were strongly advised to stop smoking
gression coefficients corresponding to the in-
and all declared to be non-smokers at the time
dependent variable Pa
2
were calculated
of prescription of oxygen. Informed consent
separately for each group and provided no
was obtained from each patient. The protocol
evidence that the Pa
2
value influenced the
of the study was approved by the ethics com-
survival of the patients in the study.
mittee of the Institute.
In the group receiving LTOT the Pa
2
while
After allocation to the control or treatment
breathing oxygen was increased in all patients
groups patients were followed closely for at
to more than 8.7 kPa (65 mmHg) (mean Pa
2
/
least three years or until death. They were
O
2
9.9 (1.1) kPa (74.0 (7.9) mmHg)). Only in
visited at home monthly by a respiratory nurse
seven patients (three of whom were survivors)
and were seen once every three months in an
was the Pa
2
increased by less than 1 kPa while
outpatient clinic by the physician responsible
breathing oxygen. The mean time spent breath-
for LTOT, being admitted to hospital for other
ing oxygen, calculated from the oxygen con-
treatment as necessary. There were no dropouts
centrator meter readings, was 13.5 (4.4) hours/
during the study. All deaths and causes of
day.
death were recorded. Each living patient was
Seventy patients died during the observation
contacted at the end of the study in December
period, 32 in the control group and 38 in
1994 by a respiratory nurse.
the LTOT group. The causes of death are
presented in table 2. Most of the deaths in both
groups were due to progression of the COPD.
Means and standard deviations of measured
The cumulative survival rate of the total
variables were calculated. Differences between
group in the first year was 88%, in the second
groups were assessed using an unpaired t test,
year 77%, and in the third year 66%. Survival
p values of <0.05 being considered statistically
significant. Survival analysis was performed
using Cox’s proportional hazards analysis for
the factors that might influence survival.
11
The
model also provides the possibility for checking
the statistical significance of the influences on
survival of one or more variables studied. The
statistical significance of the differences be-
tween two groups was assessed using Cox’s
regression model and checked by the Wilcoxon-
Mann-Whitney type non-parametric test. In
particular, the Gehan-Wilcoxon statistic was
used to confirm the lack of difference in survival
between the control and treatment groups.
Statistica and NCSS packages were used for
the computation procedures.
96
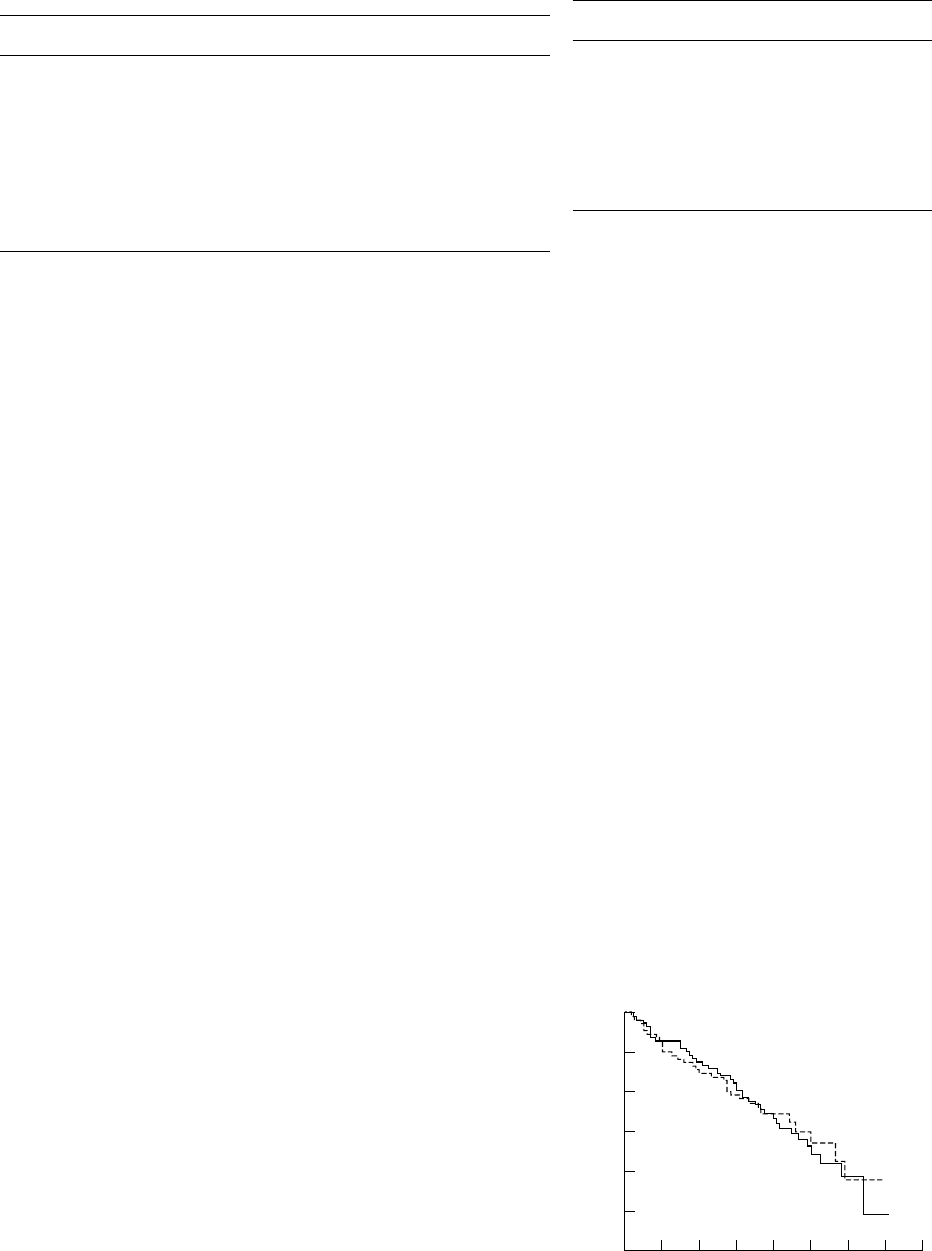
1.0
0.0
0
Survival time (months)
Cumulative survival rate
0.7
0.2
24 48 72
0.8
0.5
0.3
12 36 60 84
Controls
LTOT
Results
Figure 1 Cumulative survival rate in LTOT group and
The treatment group consisted of 103 men
controls. Difference between groups is not statistically
significant (p=0.892).
and 32 women of mean age 61.2 years (range
676 Go
´
recka, Gorzelak, S
´
liwin
´
ski, Tobiasz, Zielin
´
ski
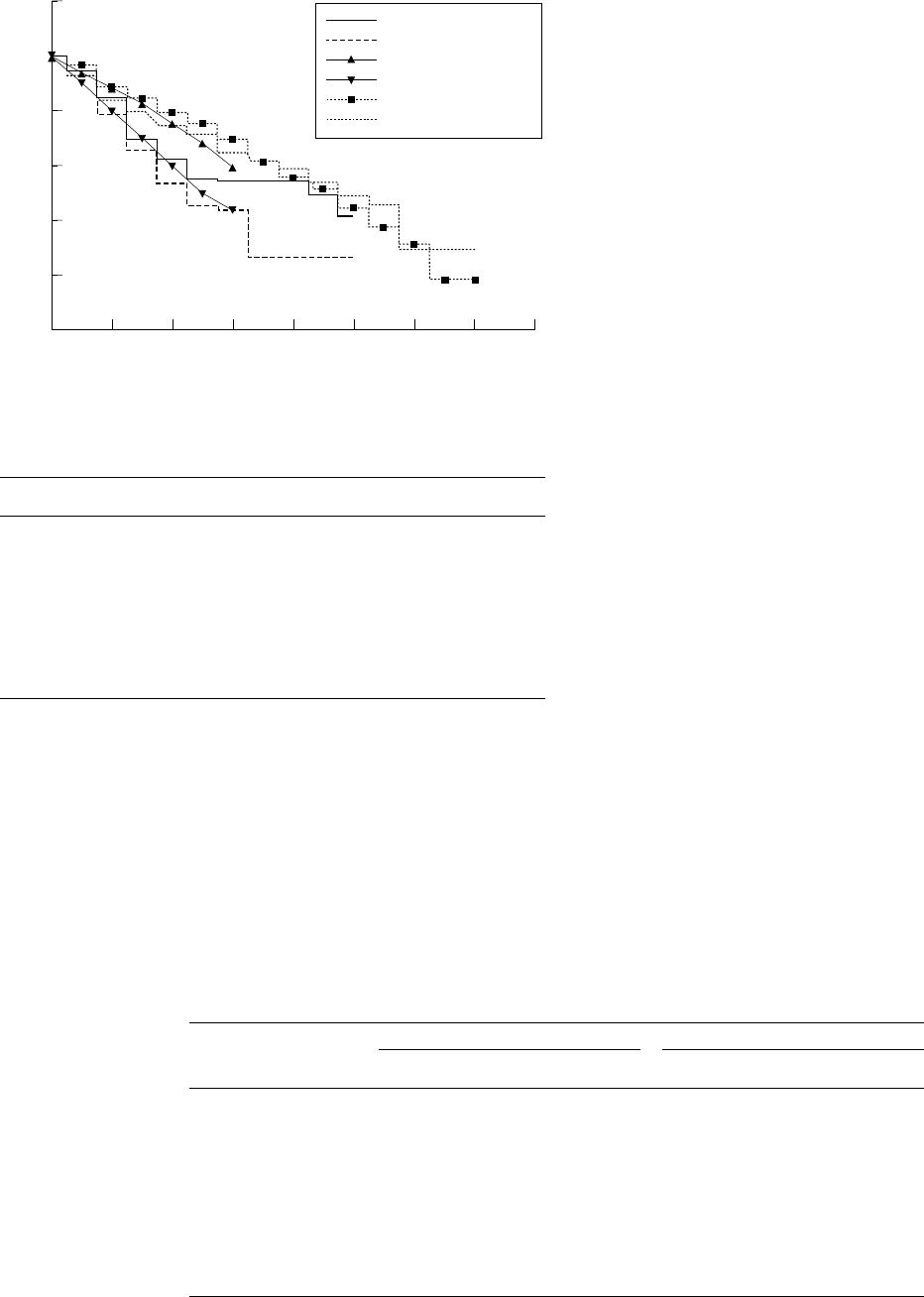
Figure 2 shows the survival curves of our
patients (in the LTOT and control groups)
superimposed on the survival curves of the
MRC and NOTT studies. The survival of our
patients with COPD with moderate hyp-
oxaemia in both the control and treatment
groups was better than of those with severe
hypoxaemia in the MRC and NOTT studies;
however, there is a considerable overlap.
The differences in the studied variables be-
tween survivors and non-survivors in the total
group are presented in table 3. Patients who
survived were significantly younger (59.4 vs
62.9 years, p=0.02), had better lung function
(VC 2.06 l vs 1.85 l, p=0.038 and FEV
1
0.89 l
vs 0.78 l, p<0.001), and higher BMI (25.1 kg/
m
2
vs 22.5 kg/m
2
, p<0.001) than non-survivors.
The differences in the studied variables be-
tween survivors and non-survivors in the con-
96
1.2
0.0
0
Survival time (months)
Cumulative survival rate
0.8
0.2
24 48 72
1.0
0.6
0.4
12 36 60 84
MRC 15 h
MRC controls
NOTT 19 h
NOTT 12 h
LTOT (this study)
Controls (this study)
trol and treatment groups separately are
Figure 2 Cumulative survival rate in the LTOT group and control patients compared
presented in table 4. In the oxygen treated
with the survival of patients in the MRC and NOTT studies.
group better lung function (VC 2.12 l in sur-
vivors vs 1.80 l in non-survivors (p=0.037)
Table 3 Mean (SD) differences in studied variables in survivors versus non-survivors in
and FEV
1
0.96 l vs 0.77 l, respectively (p=
the total study group
0.004)) and higher BMI (25.6 vs 22.6 kg/m
2
,
Variable Survivors Non-survivors p value
p=0.017) predicted better survival. In the con-
(n=65) (n=70)
trol group patients who survived were sig-
Age (years) 59.4 (8.3) 62.9 (8.4) 0.02
nificantly younger (60.8 vs 64.2 years, p<0.05)
M/F 50/15 53/17
and had higher BMI (24.6 vs 22.5 kg/m
2
,
BMI (kg/m
2
) 25.1 (4.9) 22.5 (4.3) <0.001
Pa
2
(kPa/mmHg) 8.1 (0.4)/60.5 (2.9) 8.1 (0.4)/60.4 (2.9) NS
p<0.05) than those who died.
Pa
2
(kPa/mmHg) 5.9 (0.9)/44.5 (6.8) 5.8 (0.9)/43.6 (6.7) NS
Interestingly, survivors in the treatment
VC (l) 2.06 (0.57) 1.85 (0.59) 0.038
VC (% pred) 51.0 (11.8) 47.0 (11.8) NS
group breathed oxygen for a shorter time (12.7
FEV
1
(l) 0.89 (0.30) 0.78 (0.25) <0.001
hours/day) than non-survivors (14.2 hours/
FEV
1
(% pred) 31.2 (10.4) 28.4 (9.2) NS
FEV
1
/VC (%) 42.8 (12.6) 43.1 (13.3) NS
day), although the difference was not stat-
Haematocrit (%) 47.9 (6.1) 46.5 (4.8) NS
Observation time (months) 52.0 (12.9) 30.5 (19.7) <0.001
istically significant. We have found no differ-
Steroids (no. of pts) 13 26 NS
ences in survival in patients using oxygen for
15 or more hours/day compared with those less
compliant (p=0.376). When oxygen use was
stratified there were 10 survivors and 11 non-
analysis using Cox’s regression model showed
survivors who breathed oxygen for less than 12
no differences in survival between oxygen
hours, 11 patients in each group who used
treated and control groups (fig 1). The hazard
oxygen for 12–15 hours, and only nine survivors
ratio to be a member of the control group is
compared with 16 non-survivors who breathed
equal to 0.916 with a 95% confidence interval
oxygen for more than 15 hours/day.
of 0.571 to 1.471 (the value 1, representing an
The mean Pa
2
in our patients was 8.0 kPa
equal hazard for LTOT and control groups,
(60.4 mmHg) with 74 patients having a Pa
2
is well covered by this interval). Additional
of Ζ8.0 kPa and 61 patients with a Pa
2
of
analysis using the Gehan-Wilcoxon statistic
>8.0 kPa. No differences in survival were found
with the value of −0.018 (p=0.49) confirmed
in these subgroups of patients (fig 3). We also
the lack of difference between the control and
treatment groups. found that, among the LTOT group who sur-
Table 4 Comparison of mean (SD) studied variables in survivors and non-survivors in the control and LTOT groups
Controls LTOT
Survivors Non-survivors Survivors Non-survivors
(n=35) (n=32) (n=30) (n=38)
Age (years) 60.8 (7.3) 64.2 (8.8)‡ 57.9 (9.3) 61.8 (8.0)
M/F 26/9 26/6 24/6 27/11
Pa
2
(kPa/mmHg) 8.2 (0.4)/61.2 (2.7)§ 8.2 (0.4)/61.4 (2.8) 7.9 (0.4)/59.6 (2.9) 7.9 (0.3)/59.5 (2.6)†
Pa
2
(kPa/mmHg) 5.7 (0.9)/43.1 (6.6) 5.7 (0.9)/42.5 (6.8) 6.2 (0.9)/46.2 (6.7) 5.9 (0.9)/44.6 (6.6)
VC (l) 2.00 (0.56) 1.91 (0.51) 2.12 (0.59)∗∗ 1.80 (0.66)
VC (% pred) 51.7 (12.8) 48.1 (10.1) 50.0 (10.5) 46.1 (13.2)
FEV
1
(l) 0.84 (0.32) 0.78 (0.25) 0.96 (0.27)∗∗ 0.77 (0.25)
FEV
1
(% pred) 30.6 (11.3) 29.0 (9.2) 32.2 (9.2) 28.0 (9.2)
FEV
1
/VC (%) 41.7 (13.3) 39.9 (10.8) 44.1 (11.8) 45.9 (14.6)
Haematocrit (%) 47.2 (5.8) 45.7 (4.7) 48.8 (6.5) 47.2 (4.9)
Observation time (months) 49.6 (12.8) 27.2 (19.4)‡‡ 54.8 (12.8)∗∗∗ 33.2 (19.8)
O
2
breathing time (hours) — — 12.7 (4.1) 14.2 (4.6)
BMI (kg/m
2
) 24.6 (4.6) 22.5 (4.1)‡ 25.6 (5.4)∗ 22.6 (4.5)
Steroids (no. of pts) 9 11 4 15
Pa
2
/O
2
(kPa/mmHg) 9.9 (1.3)/74.5 (9.8) 9.8 (0.9)/73.7 (6.4)
∗ p<0.05; ∗∗ p<0.01; ∗∗∗ p<0.001 survivors vs non-survivors in LTOT group.
† p<0.05 between non-survivors in both groups.
‡ p<0.05; ‡‡ p<0.01 survivors vs non-survivors in controls.
§ p<0.05 between survivors in both groups.
Effect of LTOT on survival in patients with COPD 677
96
1.0
0.0
0
Survival time (months)
Cumulative survival rate
0.8
0.2
24 48 72
0.6
0.4
12 36 60 84
BMI <20kg/m
2
BMI >25 kg/m
2
BMI 20–25
kg/m
2
96
1.0
0.0
0
Survival time (months)
Cumulative survival rate
0.8
0.2
24 48 72
0.6
0.4
12 36 60 84
Pa
O
2
<
8.0 kPa
Pa
O
2
>
8.0 kPa
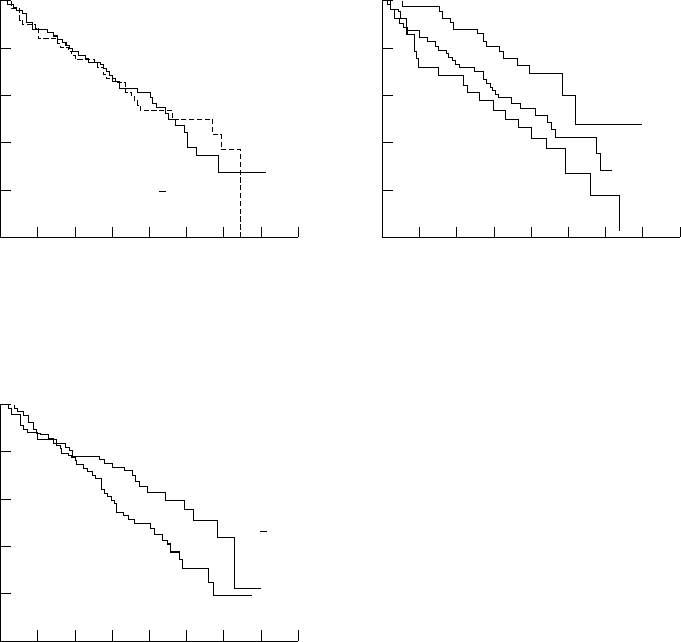
Figure 5 Cumulative survival rate in the total groupFigure 3 Cumulative survival rate in the total group
stratified for arterial oxygen tension (Pa
O
2
). No difference stratified for body mass index (BMI). Patients with BMI
>25 kg/m
2
survived significantly longer than those within survival was found in patients with Pa
O
2
Ζ8.0 kPa
and Pa
O
2
>8.0 kPa (p=0.906). BMI <20 kg/m
2
(p=0.005).
regression model with BMI and FEV
1
as in-
dependent variables were 0.992 (0.984 to
1.002) for FEV
1
and 0.942 (0.905 to 0.996)
for BMI. Additional residual regression analysis
was performed to check if BMI had a significant
influence on survival after adjusting it first for
corresponding FEV
1
. The analysis produced a
new variable – the residual values of BMI.
After taking the residual values of BMI as the
independent variable the analysis showed that
BMI still had a significant influence on survival
(p=0.05). The hazard ratio for BMI adjusted
for FEV
1
was 0.950 (95% CI 0.908 to 0.999).
The results of these analyses prove that there
96
1.0
0.0
0
Survival time (months)
Cumulative survival rate
0.8
0.2
24 48 72
0.6
0.4
12 36 60 84
FEV
1
< 0.8 l
FEV
1
>
0.8 l
is a casual relationship between FEV
1
and BMI
Figure 4 Cumulative survival rate in the total group
and that BMI is a significant predictor of sur-
stratified for forced expiratory volume in one second
vival independently of FEV
1
. No other physio-
(FEV
1
). Patients with FEV
1
[0.8 l survived significantly
longer than those with FEV
1
<0.8 l (p=0.028).
logical variable studied predicted significant
differences in survival.
vived, seven resumed smoking as judged by an
elevated carboxyhaemoglobin level. Thirty nine Discussion
The prescription of LTOT to patients withpatients (29%) used long term steroids (more
than six months), seven inhaled steroids, six COPD with moderate hypoxaemia did not pro-
long life. Moreover, within the oxygen treatedintramuscular, and 26 oral preparations. Long
term steroids were used by twice as many group no correlation was found between oxy-
gen use and survival.patients who did not survive (n=26) as sur-
vivors (n=13), but the difference did not reach The beneficial effect of LTOT in preventing
the progression of pulmonary hypertension isstatistical significance (table 3).
Using Cox’s proportional hazards analysis well known
12–14
but this treatment does not
influence the progression of the airflow lim-on the pooled sample (135 patients) we found
that the cut off value of FEV
1
of 0.8 l was itation.
15–17
To date only two controlled studies
have been reported on the effects of long termsignificantly related to survival rates (fig 4).
Survival rates also improved significantly with oxygen breathing in patients with COPD with
advanced respiratory failure – the MRC andincreasing BMI, patients with a BMI of >25 kg/
m
2
having higher survival rates than those with NOTT studies
12
– both of which found that
breathing oxygen for more than 15 hours/daya BMI of <20 kg/m
2
(fig 5). We have also found
that BMI was closely related to FEV
1
(r= substantially prolonged survival. The longer
the oxygen breathing time the better survival0.345, p<0.001). Additional analysis based on
Cox’s regression model was performed to estab- was observed.
Although the upper limit of Pa
2
for inclusionlish the influence of both parameters on sur-
vival, taking into account the positive in these studies was set at 8 kPa (60 mmHg),
most of the patients had a Pa
2
of less thancorrelation between them. The analysis with
two independent variables showed that, if BMI 7.3 kPa (55 mmHg). In the MRC study
the mean Pa
2
on air was only 6.7 kPaand FEV
1
were mutually adjusted, then only
BMI had a significant positive influence on (50.4 mmHg) for the treated group and 6.9 kPa
(51.5 mmHg) for the controls, whereas in thesurvival. Corresponding p values were 0.14 for
FEV
1
and 0.035 for BMI. Hazard ratios (95% NOTT study it was 6.8 kPa (50.8 mmHg) for
the continuous therapy group and 6.9 kPaconfidence interval) estimated by the Cox’s
678 Go
´
recka, Gorzelak, S
´
liwin
´
ski, Tobiasz, Zielin
´
ski
(51.5 mmHg) for the nocturnal oxygen group. such as those in the MRC and NOTT studies,
Mean Pa
2
in our patients was much higher
but not to our patients. There was, however,
(8.0 kPa (60.4 mmHg)). Although it might
a considerable overlap in the survival of our
have been anticipated, no differences in survival
patients and those from the abovementioned
were found in subgroups of patients with Pa
2
studies.
Ζ8 kPa and >8.0 kPa at entry to the study.
Patients receiving LTOT increased their
Our treated and control groups were well
Pa
2
on average by 2 kPa while breathing oxy-
matched at the beginning of the study. The
gen. Almost all improved their oxygenation by
only statistically significant difference (al-
at least 1 kPa, which is in accordance with the
though clinically trivial) between the groups
UK guidelines for prescribing oxygen.
23
An
was in Pa
2
which was lower in the LTOT
equal number of non-responders was found
group. This difference was probably due to a
among the survivors and non-survivors, sug-
narrow range (only 1.3 kPa (10 mmHg)) of
gesting that this factor did not influence the
inclusion Pa
2
values. Such a range restricted
survival.
the standard deviation, thereby increasing the
This comparison of our data with that in
significance of small differences in mean values.
the literature clearly confirms that survival in
Moreover, the Pa
2
did not influence the sur-
patients with COPD is influenced by airway
vival in either group or the study group as a
limitation and that LTOT prolongs life only
whole, which is the best evidence that the
when severe hypoxaemia ensues.
baseline differences were not important.
In our patients survival depended on lung
In the two landmark studies
12
survival was
function and age at entry to the study. Patients
positively associated with the number of hours
who survived had better preserved lung func-
of oxygen breathing. In our study compliance
tion and were significantly younger. In many
with the treatment was similar to that of the
previous studies the age has also proved to be
MRC trial. However, we observed no differ-
a significant predictor of survival.
4212224
ences between oxygen use in survivors (12.7
In a number of studies of patients with
hours/day) and non-survivors (14.2 hours/day).
COPD
25 26
survival was influenced by the body
When we analysed a subgroup of patients who
mass. Undernourished patients did not do so
breathed oxygen for more than 15 hours/day
well as those who were overweight
20
and this
there were more non-survivors than survivors
effect was independent of the lung function.
26
in that group. This finding may be explained
Also, our patients who survived had a sig-
by the fact that surviving patients were younger,
nificantly higher BMI than those who did not
had better lung function, and did not feel the
survive and the survival rate improved with
need to comply with the prescribed treatment
increasing body mass independently of FEV
1
,
(17 hours and more). Similarly, in a study by
as well as after adjusting BMI for FEV
1
, similar
the ANTADIR group 65% of patients with
to the results of the IPPB trial.
26
Pa
2
>8.0 kPa (60 mmHg) decreased their
We have found that, after mutually adjusting
daily oxygen use to below 15 hours because
BMI and FEV
1
, only BMI proved to have a
they found the longer treatment not necessary.
18
significant influence on survival. This may be
From two recent studies from Sweden
19
and
explained by the extremely narrow range of
the ANTADIR group in France
20
it appears that
very low FEV
1
values. However, FEV
1
proved
survival in patients with a higher prescription of
to be significantly lower in non-survivors and
oxygen is inferior to that in patients with a
the value of 0.8 l resulted in significant differ-
lower oxygen prescription and may reflect the
ences in survival in those with less and more
physician’s perception of the severity of the
advanced airway limitation.
disease.
Another factor that should be taken into
Survival in our group was similar to that of
account in studying survival is stopping smok-
patients in the IPPB trial with a similar degree
ing. It is well known that quitting smoking
of airflow limitation and no hypoxaemia.
21
Sur-
slows the decline in FEV
1
27 28
and improves the
vival in both the treatment and control groups
survival, although such an influence becomes
was better than survival of the patients in the
apparent only after approximately six years of
MRC trial
1
with more advanced airflow lim-
follow up.
29
All our patients declared to be
itation (FEV
1
0.76 l in oxygen treated group,
non-smokers when starting LTOT, however
0.63 l in controls) and more severe hypoxaemia.
seven had resumed smoking as judged by raised
It was also better than the survival of the noc-
carboxyhaemoglobin level at 1–3 months after
turnal oxygen therapy group and similar to the
entering the study. All these patients survived.
survival in the continuous oxygen treatment
The carboxyhaemoglobin level was not checked
group in the NOTT trial.
2
In a comparison of
in the control group. It is extremely difficult to
patients in the IPPB trial without hypoxaemia
draw any conclusion from this finding due to
with NOTT patients with the same degree of
the limited number of patients in whom we
airway limitation, Athonisen has found that the
could study these influences.
correction of hypoxaemia improved the survival
It has been suggested that use of long term
rates of the continuous oxygen therapy group
corticosteroids in women with COPD may ad-
to the rate of survival of patients with no blood
versely affect survival.
30
Such treatment was
gas disturbances, as opposed to the less fa-
prescribed in 29% of our patients and twice
vourable survival of the nocturnal oxygen
as many patients receiving steroids died as
group.
22
The correction of hypoxaemia in the
survived. However, this difference did not reach
MRC trial also improved the survival in oxygen
statistical significance. As we included only
treated patients compared with controls.
1
Oxy-
gen treatment was of benefit to the patients patients with COPD with fixed airway ob-
Effect of LTOT on survival in patients with COPD 679
therapy in chronic obstructive pulmonary disease. Ann
struction, the prescription of steroids reflected
Intern Med 1985;102:29–36.
13 Weitzenblum E, Sautegeau A, Ehrhart M, Mammosser M,
the severity of the disease.
Pelletier A. Long-term oxygen therapy can reverse the
We conclude that, in patients with COPD
progression of pulmonary hypertension in patients with
chronic obstructive pulmonary disease. Am Rev Respir Dis
with chronic airflow limitation but moderate
1985;131:493–6.
hypoxaemia, there is no difference in survival
14 Tobiasz M, Sliwin
˜
ski P, Hawrytkiewicz I, Patasiewicz G,
Zielin
˜
ski J. Pulmonary haemodynamics after 6 years of
rates between patients treated and not treated
oxygen therapy in COPD. Am J Respir Crit Care Med
with domiciliary oxygen. In addition, oxygen
1995;151:A255.
15 Weitzenblum E, Oswald M, Apprill M, Ratomaharo J,
use for longer periods did not improve the
Kessler R. Evolution of physiological variables in patients
survival rate. These results suggest that pre-
with chronic obstructive pulmonary disease before and
during long-term oxygen therapy. Respiration 1991;58:
scription of LTOT in this specific group of
126–31.
patients with COPD should be done more
16 Cooper CB, Howard P. An analysis of sequential physiologic
changes in hypoxic cor pulmonale during long-term oxy-
cautiously, reserving this expensive treatment
gen therapy. Chest 1991;100:76–80.
for patients with severe hypoxaemia as in the
17 Sliwin
´
ski P. Effects of long-term oxygen therapy in patients
with chronic obstructive pulmonary disease. Pneumonol
UK guidelines.
23
Alergol Pol 1992;60:20–7.
18 Barjhoux C, Pepin JL, Deschaux-Blanc C, et al. Oxy-
genotherapie au long cours a domicile. Respect de la
The authors wish to thank the following physicians from the
prescription medicale et observance d’une duree quo-
regional LTOT centres for their participation in the study:
tidienne d’au moins 15 heures. Rev Mal Resp 1994;11:
M Czajkowska-Malinowska (Bydgoszcz), A Kubica (Bystra), J
37–45.
Dobrzan
´
ska (Chełm), G Stas
´
kiewicz (Ciechano
´
w), M Fi-
19 Stro
¨
m K, Boe J. The Swedish Society of Chest Medicine:
lipowska and L Sokołowska (Krako
´
w), E Sporna (Ło
´
dz
´
), T
quality assessment and predictors of survival in long-term
Izbicka (Suwałki), M-J Pułka (Wrocław).
domiciliary oxygen therapy. Eur Respir J 1991;4:50–8.
20 Chailleux E, Binet F, Sadoul P et Commission Medico-
Technique et Sociale de L’ANTADIR. Facteurs pro-
1 Medical Research Council Working Party. Long-term dom-
nostiques de la survie des insuffisants respiratoires ob-
iciliary oxygen therapy in chronic hypoxic cor pulmonale
structifs traites par oxygenotherapie a long terme. Rev Mal
complicating chronic bronchitis and emphysema. Lancet
Resp 1992;9:603–11.
1981;i:681–6.
21 Anthonisen NR, Wright EC, Hodgkin JE, and the IPPB
2 Nocturnal Oxygen Therapy Trial Group. Continuous or
Trial group. Prognosis in chronic obstructive pulmonary
nocturnal oxygen therapy in hypoxemic chronic lung dis-
disease. Am Rev Respir Dis 1986;133:14–20.
ease: a clinical trial. Ann Intern Med 1980;93:391–8.
22 Anthonisen NR. Prognosis in chronic obstructive pulmonary
3 Skwarski K, MacNee W, Wraith PK, Sliwin
´
ski P, Zielin
´
ski
disease:results from multicenter clinical trials. Am Rev
J. Predictors of survival in patients with chronic obstructive
Respir Dis 1989;140:S95–9.
pulmonary disease treated with long-term oxygen therapy.
23 The Drug Tariff. Introduction of oxygen concentrators to the
Chest 1991;100:1522–7.
domiciliary oxygen therapy service. Publication No. FNP
4 Dubois P, Jamart J, MachielsJ, Smeets F, Lulling J. Prognosis
398. London: Department of Health and Social Security,
of severely hypoxemic patients receiving long-term oxygen
1986.
therapy. Chest 1994;105:469–74 .
24 Dallari R, Barozzi G, Pinelli G, Merighi V, Grandi P,
5 Zielin
´
ski J, Sliwin
´
ski P. Indications and methods of long-
Manzotti M, et al. Predictors of survival in subjects with
term oxygen therapy. Eur Respir Rev 1991;1:536–40.
chronic obstructive pulmonary disease treated with long-
6 Levi Valensi P, Aubry P, Donner CF, Robert B, Ruhle KH,
term oxygen therapy. Respiration 1994;61:8–13.
Weitzenblum E for the task group of SEP. Re-
25 Vanderbergh E, Van de Woestyne KP, Gyselen A. Weight
commendations for long term oxygen therapy. Eur Respir
changes in the terminal stages of chronic obstructive pul-
J 1989;2:160–4.
monary disease. Am Rev Respir Dis 1967;95:556–66.
7 Conference Report. Further recommendations for pre-
26 Wilson DO, Rogers RM, Wright EC, Anthonisen NR. Body
scribing and supplying long-term oxygen therapy. Am Rev
weight in chronic obstructive pulmonary disease. Am Rev
Respir Dis 1988;138:745–7.
Respir Dis 1989;139:1435–8.
8 Stro
¨
m K, Boe J. A national register for long-term oxygen
27 Fletcher C, Peto R. The natural history of chronic airflow
therapy in chronic hypoxia: preliminary report. Eur Respir
obstruction. BMJ 1977;1:1645–8.
J 1988;1:952–8.
28 Anthonisen NR, Connet JE, Kiley JP, et al for the Lung
9Go
´
recka D, Sliwin
´
ski P, Zielin
´
ski J. Adherence to entry
Health Study Research Group. Effects of smoking inter-
criteria and one year experience of long-term oxygen
vention and use of an inhaled anticholinergic broncho-
therapy in Poland. Eur Respir J 1992;5:848–52.
dilator on the rate of decline of FEV
1
. JAMA 1994;272:
10 Zielin
´
ski J, Sliwin
´
ski P, Tobiasz M, Go
´
recka D. Long-term
1497–505.
oxygen therapy in Poland. Monaldi Arch Chest Med 1993;
29 Postma DS, Sluiter HJ. Prognosis of chronic obstructive
48:479–80.
pulmonary disease: the Dutch experience. Am Rev Respir
11 Cox DR. Regression model and life tables. IR Stat Soc
Dis 1989;140:S100–5.
(Series B) 1972;34:187–200.
30 Stro
¨
m K. Survival of patients with chronic obstructive pul-
12 Timms RM, Khaja FU, Williams GW. The Nocturnal Oxy-
monary disease receiving long-term oxygen therapy. Am
Rev Respir Dis 1993;147:585–91.gen Trial Group. Haemodynamic response to oxygen
