
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Inhaled corticosteroids reduce the progression of airflow
limitation in chronic obstructive pulmonary disease: a meta-
analysis
E R Sutherland, H Allmers, N T Ayas, A J Venn, R J Martin
...............................................................................................................................
See end of article for
authors’ affiliations
.......................
Correspondence to:
Dr E R Sutherland, 1400
Jackson Street, J-217,
Denver, Colorado 80206,
USA; [email protected]
Received 11 April 2003
Accepted for publication
29 July 2003
.......................
Thorax 2003;58:937–941
Background: Chronic obstructive pulmonary disease (COPD) is a syndrome of chronic progressive airflow
limitation which occurs as a result of chronic inflammation of the airways and lung parenchyma. However,
the role of inhaled corticosteroids in the treatment of COPD is controversial. We hypothesised that inhaled
corticosteroids reduce the progression of airflow limitation in COPD.
Methods: A comprehensive literature search was conducted and data were analysed using random effects
methodology. The effect of inhaled steroids on annual change in forced expiratory volume in 1 second
(FEV
1
) was determined for all trials, for trials with high dose treatment regimens, and for trials in subjects
with moderate to severe airflow limitation.
Results: Data from eight controlled clinical trials of >2 years were included (n = 3715 subjects). Meta-
analysis of all study data revealed that inhaled corticosteroids reduce the rate of FEV
1
decline by 7.7 ml/
year (95% confidence interval (CI) 1.3 to 14.2, p= 0.02). Meta-analysis of studies with high dose regimens
revealed a greater effect of 9.9 ml/year (95% CI 2.3 to 17.5, p = 0.01) compared with the meta-analysis
of all studies.
Conclusions: Inhaled corticosteroid treatment for >2 years slows the rate of lung function decline in COPD.
The effect observed with high dose regimens is greater than that with all regimens combined. These data
suggest a potential role for inhaled corticosteroids in modifying the long term natural history of COPD.
C
hronic obstructive pulmonary disease (COPD) is a
syndrome of chronic and progressive airflow limitation
which occurs as a result of chronic inflammation of the
airways and lung parenchyma.
1
Chronic inflammation leads
to a progressive deterioration of airflow which is manifested
by an accelerated annual rate of decline in the forced
expiratory volume in 1 second (FEV
1
) of approximately
60 ml/year.
2
In comparison, non-smokers experience a rate
of decline in FEV
1
of approximately 30 ml/year.
2
This
physiological deterioration provides the substrate for the
clinical manifestations of COPD, which include cough,
sputum production and dyspnoea. Therapeutic options to
modify disease progression in COPD are limited and,
although pharmacological interventions such as inhaled
bronchodilators effectively treat symptoms, they have not
been shown to modify the long term progression of airflow
limitation.
Inhaled corticosteroids reduce airway inflammation, air-
flow limitation, and symptoms in asthma and are the
mainstay of treatment for patients with persistent asthma.
3
In COPD, however, the role of inhaled corticosteroids is
controversial, as the inflammatory phenotype differs from
that seen in asthma
1
and has been reported to respond less
favourably in the short term to inhaled corticosteroids.
4
There
is also ongoing debate surrounding the importance of the
physiological versus the clinical response to inhaled corticos-
teroids. Individual controlled clinical trials comparing
inhaled corticosteroids with placebo for treatment periods
of 12 months or more have failed to show a significant effect
on the rate of decline in FEV
1
. However, these same trials
have shown that medium to high dose
3
inhaled corticoster-
oids have beneficial effects on clinical outcomes such as
symptoms,
5
exacerbation rate, and health status.
67
The fact
that these drugs have a significant clinical effect even though
their physiological effects are negligible may explain why
inhaled corticosteroids are prescribed frequently for the
treatment of COPD.
8
We hypothesised that inhaled corticosteroids reduce the
progression of airflow limitation in COPD, but that the ability
of previous trials to detect a numerically small long term
effect on FEV
1
has been limited by design considerations
including sample size and duration of follow up. To minimise
the effect of the early increase in FEV
1
seen in many trials of
inhaled steroids in COPD, this meta-analysis focused on trials
with a duration of >2 years. The primary outcome measure
for this meta-analysis was progression of airflow limitation as
reflected by annual decline in FEV
1
.
METHODS
Data sources and study selection
A comprehensive search of the published literature was
conducted using the medical subject headings chronic
obstructive pulmonary disease, chronic bronchitis, pulmon-
ary emphysema, steroids, beclomethasone, budesonide, and
triamcinolone and the supplementary terms flunisolide and
fluticasone. The search was restricted to clinical trials and
MEDLINE (1966–February 2003 (week 2)), CINAHL (1982–
February 2003 (week 2)), International Pharmaceutical
Abstracts (1970–February 2003 (week 2)), and the
Cochrane controlled trials register (fourth quarter, 2002)
were queried. Reference lists from retrieved articles were
reviewed to identify additional candidates for inclusion.
Attempts were made to identify additional data or unpub-
lished studies of inhaled corticosteroids in COPD through
discussions with experts in the area of COPD pharmacother-
apy at the 2002 American Thoracic Society international
meeting.
Included studies met the following criteria: (1) design:
randomised controlled clinical trial of an inhaled corticoster-
oid in subjects with COPD; (2) follow up: minimum of 1 year;
937
www.thoraxjnl.com
(3) primary outcome variable: change in FEV
1
over time; (4)
disease-specific factors: subjects with asthma were excluded
and subjects were studied when the disease was stable; and
(5) publication type: not published solely in abstract form.
The results of the literature search were then sorted, using
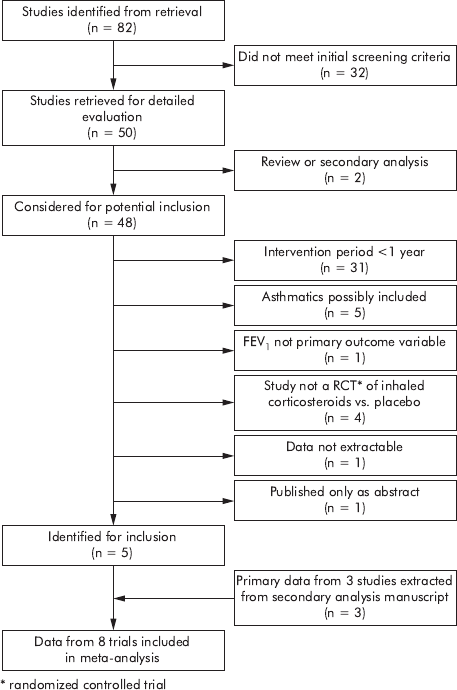
the criteria above, for inclusion in the meta-analysis (fig 1).
Pertinence of these citations to the meta-analysis was
evaluated using a sequential screening approach beginning
with the title, followed by evaluation of the abstract and then
the paper itself. Screening of citations was performed
individually by each author and trials were selected and
agreed upon by consensus.
Outcome variable
The primary outcome for this meta-analysis was annual rate
of change in FEV
1
, a primary end point for which multiple
individual trials of inhaled corticosteroid therapy have been
unable to show a significant effect.
Data extraction and quality
Data regarding the primary outcome variable were abstracted
from each article and confirmed by consensus. With one
exception,
9
investigators modelled the mean annual change
in FEV
1
to take into account the correlated nature of repeated
measures within individuals. One of the eight trials
10
reported
a median change in FEV
1
over time. After an unsuccessful
attempt to obtain mean values for the primary outcome from
this large study (n = 1277), an assumption was made that the
median value approximated the mean, and standard error
was estimated from the reported p value
11
for the median
differences. The assumed mean and estimated standard error
conformed closely to outcome data reported in other clinical
trials. Standard error was calculated from the 95% confidence
interval where necessary.
12
Data reflecting change over the
entire study period were abstracted for use in the meta-
analysis.
Statistical analysis
The random effects model of DerSimonian and Laird
13
was
used to perform quantitative synthesis of the extracted data.
Random effects methodology was chosen to account for both
within study and between study variation.
14
Summary effect
estimates were represented as a point estimate and 95%
confidence intervals and plotted on a forest plot.
15
Heterogeneity of data was evaluated using the Q statistic.
16
Publication bias was evaluated by means of a funnel plot
17
and formal statistical analysis.
18
STATA software version 7
(STATA Corporation, College Station, Texas) was used for all
analyses.
RESULTS
Study selection
The literature search strategy identified 82 unique and
potentially relevant citations (fig 1). Review of bibliographies
and discussion with experts did not uncover additional
studies. Thirty two citations did not meet initial screening
criteria, leaving 50 citations for which abstracts were
reviewed for inclusion. Of these abstracts, one was a
qualitative review of inhaled corticosteroids in COPD and
one was a secondary pooled analysis of primary data from
three prior clinical trials. Of the remaining 48 abstracts, 43
were excluded for the following reasons: follow up was less
than 1 year (n = 31), subjects with asthma were possibly
included (n = 5), FEV
1
was not the primary outcome variable
(n = 1), study was not a randomised controlled clinical trial
of inhaled corticosteroids versus placebo (n = 4), data were
not extractable (n = 1), or the study was published in
abstract form only (n = 1).
The five remaining studies
5791012
were included in this
meta-analysis. Close evaluation of the secondary analysis
paper
19
revealed it to be an analysis of data from three prior
clinical trials of long term inhaled corticosteroids
20–22
in
which, in some cases, a mixed population of subjects with
asthma and COPD were evaluated. The authors obtained the
original study data for subjects with COPD and performed a
pooled analysis of the data from these subjects.
19
Because of
their pertinence to this meta-analysis, these data were
included, making data from subjects enrolled in a total of
eight individual studies available for meta-analysis.
Subject and study characteristics
Data for 3715 subjects were available for meta-analysis.
Table 1 reports age, baseline FEV
1
as percentage predicted,
FEV
1
percentage reversibility in response to inhaled beta-
agonist, and smoking prevalence for the placebo and steroid
arms of each study. Table 2 reports the number of subjects by
treatment allocation, drug used for treatment, and duration
of treatment for each study. Although the search strategy was
designed to identify studies with a duration of as little as 1
year, all studies were >2 years in duration. The annual
change in the rates of FEV
1
decline from each study is
reported in table 3.
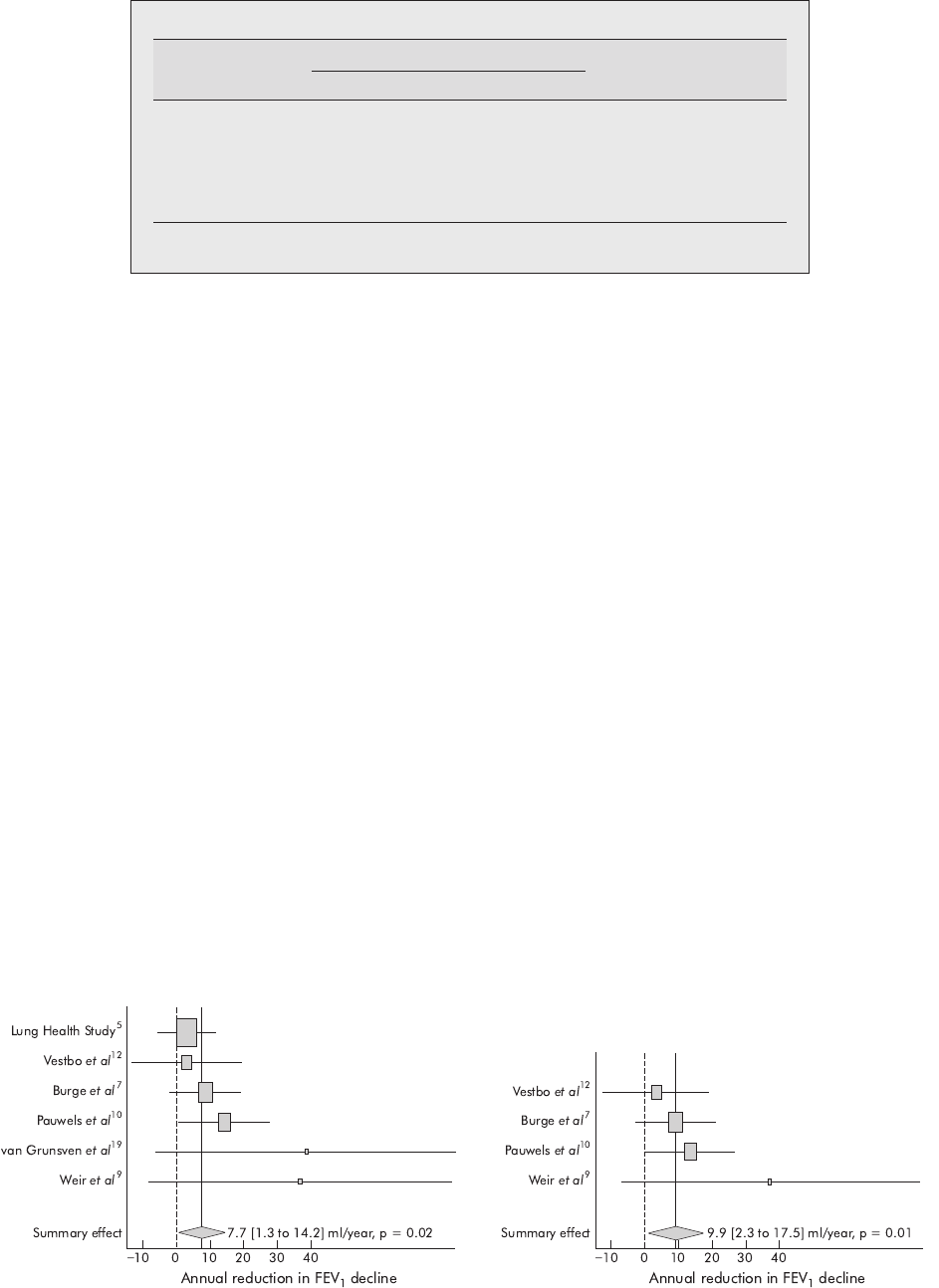
Effect of inhaled corticosteroids on FEV
1
Random effects meta-analysis of data from all studies
indicated that inhaled corticosteroids significantly reduced
the rate of decline in FEV
1
by 7.7 ml/year (95% confidence
interval (CI) 1.3 to 14.2, p = 0.02; fig 2).
Figure 1 Flow diagram depicting the selection of studies for meta-
analysis.
938 Sutherland, Allmers, Ayas, et al
www.thoraxjnl.com

Sensitivity analyses were performed to determine whether
there was an increased response to high dose
3
inhaled
corticosteroids and whether inhaled steroids had a greater
effect in subjects with baseline FEV
1
(50% of predicted.
Meta-analysis of trials with high dose steroid regimens
(n = 2416)
791012
demonstrated a greater reduction in the rate
of FEV
1
decline of 9.9 ml/year (95% CI 2.3 to 17.5, p = 0.01)
than was seen in the meta-analysis of all studies (fig 3).
Comparison meta-analysis of studies with lower dose regi-
mens could not be performed as only one of the included
studies (the Lung Health Study
5
) used a non high-dose
regimen. The secondary analysis of van Grunsven and
colleagues
19
pooled data from subjects who were treated
with a mixture of medium and high dose regimens and these
data were therefore not appropriate for inclusion in the
analysis of lower dose studies.
In studies which enrolled subjects with a baseline FEV
1
of
(50% predicted (n = 1032 subjects),
7919
a reduction in the
rate of decline in FEV
1
of 18.3 ml/year was observed, but the
95% confidence interval was not significant (21.5 to 38.0 ml/
year, p = 0.07).
Statistical heterogeneity and publication bias
For the analysis of all studies there was no evidence of
significant statistical heterogeneity (Q = 5.9, p = 0.32).
Furthermore, there was no evidence of significant statistical
heterogeneity in the analysis of studies which used high dose
inhaled corticosteroid regimens (Q = 2.4, p = 0.50) or in the
analysis of studies which enrolled subjects with a baseline
FEV
1
of (50% predicted (Q = 2.8, p = 0.25).
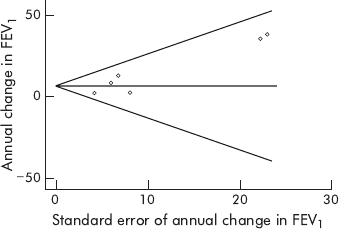
Funnel plot analysis (fig 4)
17
of the included studies
demonstrated asymmetry with an Egger
18
p = 0.03, suggest-
ing that there was publication bias manifested by an absence
in the literature of studies that resulted in negative mean
effect estimates with high standard errors.
DISCUSSION
The results of this meta-analysis suggest that inhaled
corticosteroids significantly slow the rate of deterioration in
FEV
1
in patients with COPD when used for a period of at least
24 months. This effect appears to be augmented by high dose
regimens
3
of inhaled corticosteroids.
The size of the effect derived from inhaled corticosteroids is
numerically small, with the reduction in the rate of FEV
1
decline ranging from 7.7 to 9.9 ml/year. Although these
numbers are small in the absolute, they represent a relative
reduction in the rate of FEV
1
decline of approximately 13–
17% in smokers with COPD and 26–33% in non-smokers with
COPD.
2
This effect of inhaled corticosteroids on the rate of
FEV
1
decline is less than the effect of smoking cessation,
which can achieve a reduction of approximately 50% in the
rate of deterioration.
2
However, many of the subjects
contributing data to this meta-analysis continued to smoke
during inhaled corticosteroid treatment, suggesting that the
beneficial effect of inhaled corticosteroids occurs despite an
ongoing inflammatory stimulus.
Current guidelines recommend that inhaled corticosteroids
be administered to COPD patients with frequent symptoms
despite optimal bronchodilator therapy, frequent exacerba-
tions, and FEV
1
of (50% predicted.
23
Analysis of studies on
this population showed a trend towards a greater effect that
did not achieve statistical significance. Further research is
required to determine which patients respond best to inhaled
corticosteroids and whether this effect is greater in patients
with low FEV
1
. Although we were able to show a larger effect
size with studies of high dose regimens compared with all
studies, only one lower dose study
5
was available for
comparison, preventing a comparison meta-analysis and
possibly limiting the ability to draw conclusions about
dose-response. The majority of long term studies of inhaled
corticosteroids in COPD have used high dose regimens and,
although the greater effect size seen in high dose studies may
Table 1 Age, physiological characteristics, and smoking status of study subjects randomised to receive placebo or inhaled
corticosteroid
Study
Age FEV
1
(% predicted) % Reversibility % Smokers
Placebo Steroid Placebo Steroid Placebo Steroid Placebo Steroid
Burge et al
7
63.8 (7.1) 63.7 (7.1) 50.0 (14.9) 50.3 (14.9) 4.4 (3.4) 4.4 (3.5) 39.2 36.4
Lung Health Study Research
Group
5
56.4 (6.8) 56.2 (6.8) 67.2 (12.7) 68.5 (12.8) 6.8 (7.7) 6.5 (7.3) 89.8 90.5
Pauwels et al
10
52.4 (7.7) 52.5 (7.5) 76.9 (13.2) 76.8 (12.4) 2.8 (3.6 ) 2.9 (3.8) 100 100
van Grunsven et al
19
61 (7) 61 (7) 44.0 (10.0) 46.0 (11.0) 2.9 (2.5 ) 3.2 (2.7) 39 34
Vestbo et al
12
59.1 (9.7) 59.0 (8.3) 86.9 (21.1) 86.2 (20.6) 7.2 (9.4 ) 8.1 (8.9) 77.2 75.9
Weir et al
9
* 67.6 (1.0) 65.5 (1.0) 41.4 (16.0) 39.7 (14.0) 11.5 11.2 42.9 34.7
Data reported as mean (SD) except *mean (SE).
Calculated from data reported in paper, absolute change 130 ml in placebo group and 120 ml in steroid group.
Table 2 Number of subjects and treatment allocation by study
Study
N
Drug
Duration of
treatmentPlacebo Steroid
Burge et al
7
375 376 Fluticasone, 500 mg bid 36 months
Lung Health Study
Research Group
5
557 559 Triamcinolone, 600 mg bid 40 months
Pauwels et al
10
643 634 Budesonide, 400 mg bid 36 months
van Grunsven et al
19
88 95 Beclomethasone, 800 mg or 1500 mg qd,
or budesonide, 800 mg bid
24–30 months
Vestbo et al
12
145 145 Budesonide, 800 mg qam/400 mg qpm 6
6 months, then 400 mg bid
36 months
Weir et al
9
49 49 Beclomethasone, 750 mg (if weight ,50 kg)
or 1000 mg bid (if weight >50 kg)
24 months
Effect of inhaled corticosteroids on progression of airflow limitation in COPD 939
www.thoraxjnl.com
be evidence of a dose-response effect, it may also reflect
issues related to sample size and statistical heterogeneity.
The clinical importance of an improvement in FEV
1
of 7.7–
9.9 ml/year is debatable. However, as noted previously, many
of the trials included in this meta-analysis showed a benefit
of inhaled corticosteroids with regard to secondary outcome
measures such as exacerbation frequency, symptom scores,
and quality of life. Exacerbations contribute to the decline in
lung function in COPD,
24
and the effect of inhaled steroids on
FEV
1
may be mediated in part by a reduction in exacerbation
frequency. Additionally, the physiological benefit of inhaled
corticosteroids may not be reflected by changes in FEV
1
but
rather by changes in lung volumes and hyperinflation, as
occurs with inhaled bronchodilators.
25
Although the numer-
ical effect of inhaled steroids is small, these drugs do have a
moderate relative effect and may supplement other interven-
tions such as smoking cessation in modifying the natural
history of this disease. The fact that studies with a follow up
of >2 years were included in this meta-analysis may
underestimate the beneficial short term effects of inhaled
steroids. In many clinical trials of these drugs in COPD there
is an increase in FEV
1
over the initial months of treatment, so
we chose long term studies to avoid an undue influence of
this early increase on the long term outcome. However, we
did not have access to the primary data necessary formally to
test the effect of this initial increase on the overall outcome,
and further studies are needed to determine the effect of this
initial increase on the overall response.
The choice of primary end points also affects interpretation
of clinical trials of inhaled corticosteroids in COPD, and
improving FEV
1
has been an elusive goal in clinical trials in
patients with this condition. FEV
1
is poorly correlated with
symptom indices,
23
and improvement in FEV
1
may be a
suboptimal choice of primary outcome for clinical trials.
Many of the trials included in this meta-analysis reported
significant results for secondary end points including
symptom scores, quality of life and exacerbation rates, an
effect confirmed in a recent meta-analysis of exacerbation
rates in short and long term trials of inhaled corticosteroids
in COPD.
26
It should be noted that, in seven of the eight
included studies, a modelled rather than crude estimate of
post-bronchodilator FEV
1
change was reported. Although
this may have improved our ability to detect a positive effect,
modelling is a necessary and appropriate means of account-
ing for the correlated nature of longitudinal spirometric data.
This meta-analysis has limitations. Although we did not
find any evidence of statistical heterogeneity, there is design
heterogeneity between the included studies with regard to
factors such as pre-randomisation physiology, smoking
prevalence, drug dosing, and study duration. This is likely
to have an impact on inhaled corticosteroid efficacy, but it
may also more fairly represent the real life clinical variation
seen in the treatment of patients with COPD. However, our
inclusion of only randomised studies forced these factors to
be distributed randomly in the study population, reducing
the likelihood that they were significant confounders. There
is a suggestion of publication bias in that there is an absence
of small studies with negative outcomes in the reported
literature. Whether this represents true publication bias or
whether studies with such results have been performed is
unclear. There is no shortage of large studies with negative
outcomes, and it is unlikely that identifying small negative
studies would significantly impact on these results. Finally,
as is often the case in meta-analyses of published literature,
data quality differed between studies. For example, only 72%
of included data were specified by original investigators to
have been analysed by intent-to-treat principles, and median
(rather than mean) outcome data were used in one case.
Table 3 Annual rate of decline in post-bronchodilator FEV
1
by treatment allocation
Study
Annual change in FEV
1
(ml/year)
Reduction in annual change
in FEV
1
(ml/year)Placebo Steroid
Burge et al
7
259 (4.4) 250 (4.1) 9.0 (6.0)
Lung Health Study
Research Group
5
247.0 (3.0) 244.2 (2.9) 2.8 (4.2)
Pauwels et al
10
260 246.7 13.3 (6.8)
van Grunsven et al
19
NR NR 39.0 (23.0)
Vestbo et al
12
249.1* 246.0* 3.1 (8.1)
Weir et al
9
256.9 (15) 220.6 (16) 36.3 (22.3)
All data mean (SE) except where SE not reported or calculable (*) or where median values reported (, see text)
NR = not reported; FEV
1
= forced expiratory volume in 1 second.
Figure 2 Summary effect of inhaled corticosteroids on the rate of
decline in post-bronchodilator FEV
1
in patients with COPD. The centre of
the diamond indicates the summary effect and its width the 95%
confidence interval.
Figure 3 Summary effect of high dose inhaled corticosteroids on the
rate of decline in post-bronchodilator FEV
1
in patients with COPD. The
centre of the diamond indicates the summary effect and its width the 95%
confidence interval.
940 Sutherland, Allmers, Ayas, et al
www.thoraxjnl.com
In summary, these data suggest that inhaled corticoster-
oids significantly slow the rate of decline in FEV
1
in patients
with COPD. The effect is numerically small but represents a
moderate relative effect compared with interventions such as
smoking cessation. Further clinical data from studies of
inhaled corticosteroids are necessary to inform clinicians
more fully as to the appropriate place of these drugs in COPD
pharmacotherapy.
ACKNOWLEDGEMENTS
The authors acknowledge the contribution of Michael A Stoto, PhD
to this research.
Authors’ affiliations
.....................
E R Sutherland, R J Martin, Department of Medicine, National Jewish
Medical and Research Center and the University of Colorado Health
Sciences Center, Denver, Colorado, USA
H Allmers, Department of Dermatology, Environmental Medicine and
Health Sciences, University of Osnabrueck, Osnabrueck, Germany
N T Ayas, Respiratory Division, Department of Medicine, Vancouver
General Hospital, and the Centre for Clinical Epidemiology and
Evaluation, Vancouver, Canada
A J Venn, Division of Epidemiology and Public Health, University of
Nottingham, Nottingham, UK
Funding: NIH K23 HL04385 (Dr Sutherland), The Wellcome Trust
(Dr Venn).
REFERENCES
1 Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med
2000;343:269–80.
2 Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung
Health Study participants after 11 years. Am J Respir Crit Care Med
2002;166:675–9.
3 National Heart, Lung and Blood Institute. Guidelines for the diagnosis and
management of asthma. Bethesda, MD: National Heart, Lung and Blood
Institute, 1997.
4 Culpitt SV, Maziak W, Loukidis S, et al. Effect of high dose inhaled steroid on
cells, cytokines, and proteases in induced sputum in chronic obstructive
pulmonary disease. Am J Respir Crit Care Med 1999;160(5 Pt 1):1635–9.
5 The Lung Health Study Research Group. Effect of inhaled triamcinolone on the
decline in pulmonary function in chronic obstructive pulmonary disease.
N Engl J Med 2000;343:1902–9.
6 Spencer S, Calverley PM, Sherwood Burge P, et al. Health status deterioration
in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care
Med 2001;163:122–8.
7 Burge PS, Calverley PM, Jones PW, et al. Randomised, double blind, placebo
controlled study of fluticasone propionate in patients with moderate to severe
chronic obstructive pulmonary disease: the ISOLDE trial. BMJ
2000;320:1297–303.
8 Jackevicius CA, Chapman KR. Prevalence of inhaled corticosteroid use among
patients with chronic obstructive pulmonary disease: a survey. Ann
Pharmacother 1997;31:160–4.
9 Weir DC, Bale GA, Bright P, et al. A double-blind placebo-controlled study of
the effect of inhaled beclomethasone dipropionate for 2 years in patients with
nonasthmatic chronic obstructive pulmonary disease. Clin Exp Allergy
1999;29(Suppl 2):125–8.
10 Pauwels RA, Lofdahl CG, Laitinen LA, et al. Long-term treatment with inhaled
budesonide in persons with mild chronic obstructive pulmonary disease who
continue smoking. European Respiratory Society Study on Chronic Obstructive
Pulmonary Disease. N Engl J Med 1999;340:1948–53.
11 Greenland S. Quantitative methods in the review of epidemiologic literature.
Epidemiol Rev 1987;9:1–30.
12 Vestbo J, Sorensen T, Lange P, et al. Long-term effect of inhaled budesonide in
mild and moderate chronic obstructive pulmonary disease: a randomised
controlled trial. Lancet 1999;353:1819–23.
13 DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials
1986;7:177–88.
14 Sutton AJ, Abrams KR, Jones DR, et al. Random effects methods for combining
study estimates. Methods for meta-analysis in medical research. Chichester:
John Wiley & Sons, 2000:73–86.
15 Light RJ, Singer JD, Willett JB. The visual presentation and interpretation of
meta-analysis. In: Cooper H, Hedges LV, eds. The handbook of research
synthesis. New York: Russell Sage Foundation, 1994:439–54.
16 Cochran WG. the combination of estimates from different experiments.
Biometrics 1954;10:101–29.
17 Begg CB, Mazumdar M. Operating characteristics of a rank correlation test
for publication bias. Biometrics 1994;50:1088–101.
18 Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected
by a simple, graphical test. BMJ 1997;315:629–34.
19 van Grunsven PM, van Schayck CP, Derenne JP, et al. Long term effects of
inhaled corticosteroids in chronic obstructive pulmonary disease: a meta-
analysis. Thorax 1999;54:7–14.
20 Derenne JP. Effects of high-dose inhaled beclomethasone on the rate of
decline in FEV
1
in patients with chronic obstructive pulmonary disease: results
of a 2 year prospective multicentre study. Am J Respir Crit Care Med
1995;151:A463.
21 Kerstjens HA, Brand PL, Hughes MD, et al. A comparison of bronchodilator
therapy with or without inhaled corticosteroid therapy for obstructive airways
disease. Dutch Chronic Non-Specific Lung Disease Study Group. N Engl J Med
1992;327:1413–9.
22 Renkema TE, Schouten JP, Koeter GH, et al. Effects of long-term treatment with
corticosteroids in COPD. Chest 1996;109:1156–62.
23 Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the
diagnosis, management, and prevention of chronic obstructive pulmonary
disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung
Disease (GOLD) workshop summary. Am J Respir Crit Care Med
2001;163:1256–76.
24 Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between
exacerbation frequency and lung function decline in chronic obstructive
pulmonary disease. Thorax 2002;57:847–52.
25 Boni E, Corda L, Franchini D, et al. Volume effect and exertional dyspnoea
after bronchodilator in patients with COPD with and without expiratory flow
limitation at rest. Thorax 2002;57:528–32.
26 Alsaeedi A, Sin DD, McAlister FA. The effects of inhaled corticosteroids in
chronic obstructive pulmonary disease: a systematic review of randomized
placebo-controlled trials. Am J Med 2002;113:59–65.
Figure 4 Funnel plot of included studies.
Effect of inhaled corticosteroids on progression of airflow limitation in COPD 941
www.thoraxjnl.com
