
A Practical Guide to Writing a
Ruth L. Kirschstein NRSA Grant
Second Edition
Andrew D. Hollenbach, Ph.D.
Professor, Department of Genetics
Louisiana State University Health Sciences Center
New Orleans, LA, United States

PREFACE FOR THE SECOND EDITION
It has been nearly 5 years since I completed the first edition of this
book. In that time, I have helped a large number of trainees to con-
struct a Ruth L. Kirschstein training grant. At the same time, my
grant-writing seminar expanded from a single, 1-h seminar to an inter-
active 6-h seminar covered in two, 3-h sessions. Through both of these
services, I’ve interacted with many trainees and their mentors,
I’ve answered questions that may be specific to their situation or may
be more general in nature. Regardless, I realized through these ques-
tions that there were topics and concerns that I hadn’t even considered
or that I unintentionally omitted when writing the first edition.
Further, some of the questions highlighted the fact that I didn’t discuss
certain topics in enough detail. Therefore, I felt the need to include
these issues and provide clarity in what I had previously written.
More importantly, the National Institutes of Health (NIH) have
made several significant changes in the Ruth L. Kirschstein training
grant application packet since the first edition of this book was pub-
lished. Some of these changes involved general topics made to all NIH
grant application (e.g., Biosketch), others were changes that specifi-
cally applied to these training grants (e.g., submission of References,
etc.), and some are newly required sections (i.e., Institutional
Environment and Commitment to Training). Regardless of whether
these changes or additions were general or specific, they were signifi-
cant enough that parts of the first edition of this book were obsolete.
In the second edition I have expanded and updated the original ver-
sion to include all of these topics. Specifically, I describe the combining
of previous sections (Doctoral Dissertation and Other Research
Experience, Goals for Training, and Activities Planned) into a new
section entitled “Applicant’s Background and Goals for Fellowship
Training.” I discuss how an applicant or sponsor’s productivity is now
illustrated through a new subsection of the Biosketch call
“Contributions to Science.” I include information on the newly
required section “Description of Institutional Environment and
Commitment to Training” and where appropriate I provide more
detail on how to tailor your writing style to the type of grant you are
applying for (i.e., F30, F31, or F32). Further, I highlight the impor-
tance of providing a clinical slant to the overall training of MD/PhD
students applying for the F30 mechanism.
In addition to these major changes, I also expanded discussions on
issues that may be considered minor, but yet are important to consider
when constructing a training grant. This information includes updating
the process by which References submit their recommendations and
providing information on formatting issues to improve the overall
visual image of the grant application (i.e., including “white space” and
care in the use of acronyms). Finally, through these 5 years I found
that many times, trainees had little to no understanding of the basics
of the NIH, including the overall structure of the NIH, the common
granting mechanisms available through the NIH, and the individuals
at the NIH who assist in the review process and the postreview award-
ing of the applications. Therefore, I have included a brief description
of these topics.
I never deluded myself into thinking that my book would become a
New York Times bestseller, nor that I would get rich and retire off of
the royalties. Honestly, fame and fortune have never been driving fac-
tors in my career and life choices that I make. What I set out to do
5 years ago when I sat down to work on the first edition of this book
was to write something that I knew was needed, a resource that I knew
would educate trainees and mentors on the ins and outs of constructing
a training grant, and a guide that I hoped would contribute to trainees
being successful in garnering important and prestigious funding.
Through word of mouth, from direct interactions with trainees, and
from reading reviews of my book on Amazon.com (which my Mom
checks on a regular basis!) I know that in that respect I have been suc-
cessful. It is my sincere hope that this second edition, updated to reflect
changes in the Ruth L. Kirschstein grant application package, con-
tinues to do what I originally set out to do.
Andrew D. Hollenbach, Ph.D.
January 2018
x Preface for the Second Edition
A Practical Guide to Writing a
Ruth L. Kirschstein NRSA Grant

CHAPTER
1
1
Ruth L. Kirschstein—The Woman and Her
Legacy
1.1 RUTH L. KIRSCHSTEIN—A BRIEF BIOGRAPHY
The Ruth L. Kirschstein National Research Service Award (NRSA)
training grants from the National Institutes of Health (NIH) are one
of the most prestigious training awards given to predoctoral students
and postdoctoral researchers in the United States. However, very few
people actually know who Ruth L. Kirschstein was, what she accom-
plished in her career, and why these grants serve as her ongoing legacy
to scientific training. Ruth Lillian Kirschstein, born in 1926, was the
daughter of immigrants fleeing Jewish persecution in Russia whose
original name, now forgotten, was changed to Kirschstein by a tired
Ellis Island immigration official. She was raised in Brooklyn, the
daughter of two teachers who instilled a love of learning in Ruth
through constant exposure to education and culture. As a result of the
continual discrimination against Jews at the time, her parents encour-
aged Ruth to pursue her own interests in life, regardless of societal atti-
tudes. Therefore, Ruth never realized or accepted that there was
nothing that she could not accomplish once she set her mind to it. This
familial environment also instilled high personal standards of excel-
lence in Ruth. Although classically trained and accomplished in play-
ing the French horn, she realized that her talent was limited and would
not allow her to achieve the level of professional excellence that she
desired. Therefore, she decided to follow her second love and pursue a
career in medicine.
Ruth enrolled in Long Island University in 1943 and after complet-
ing college in 1947 applied to medical schools across the country.
During this process, she fought gender and ethnic discrimination as a
Jewish female, a bias that further exasperated the difficulty of being
accepted into medical schools because of the quota system for admit-
ting Jews to professional training programs. Not accepting defeat, she
persevered and was finally accepted and enrolled at Tulane University
A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant. DOI: https://doi.org/10.1016/B978-0-12-815336-9.00001-6
© 2018 Elsevier Inc. All rights reserved.
Medical School in New Orleans, Louisiana, becoming the only student
from her Long Island University graduating class to be accepted into
medical school. In a class of 109 new medical students, Ruth was one
of only 10 women and the only Jewish female enrolled that year. She
fully dedicated herself to becoming a doctor, eventually becoming
interested in the study of disease and the effects of disease on the
human body, which ultimately directed her to a career in pathology.
After completing medical school, Ruth elected to perform her year-
long internship in medicine and surgery at Kings County Hospital in
Brooklyn, New York. This decision was made partly so that she could
be near her new husband, Al Rabson, who was pursuing his own
internship in New York, but also partly because of Kings County
Hospital’s humanitarian mission. Kings County Hospital was, and still
is, dedicated to providing care to all people regardless of their ability
to pay, an attention to social justice that appealed to Ruth and
influenced her entire career. During her internship, she was exposed to
diseases and infections of all kinds, including tuberculosis, which she
contracted and laid dormant in her for years. More importantly, this
time in her career exposed her to many different aspects of medicine,
training her to become adept at making on-the-spot decisions. A resi-
dency in pathology at Providence Hospital in Detroit followed Ruth’s
internship until her husband was accepted into the pathology residency
program in New Orleans. When Tulane University invited her to
continue her pathology training, Ruth accepted.
A year later, in 1955, Ruth and her husband moved from New
Orleans to Bethesda, Maryland, where Al accepted a research position
in the National Cancer Institute (NCI) and Ruth completed a second
year of pathology residency. Despite balancing work, home, and a
newborn son, Ruth maintained a positive attitude and an incredible
enthusiasm, serving as an excellent parent and role model for their
son. At this time Ruth also began fighting for the rights of those com-
monly discriminated against. Her parents had always stressed and
enforced the importance of social justice in their children, an awareness
that was later influenced by Franklin D. Roosevelt’s fireside chats in
which he made a call to help those in need. Further, her firsthand
experience of racial segregation in the Deep South emboldened her
desire to fight against the inequity of segregation and discrimination.
Ruth brought this desire with her to the NIH where she fought for pay
2 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
rises for deserving women and minorities in a system and at a time
when these merit-based increases were not common.
During her residency, in addition to her clinical duties, Ruth would
sometimes perform research studies with her husband. She had the
chance to test out new instruments, such as the Coulter counter, which
was invented at the NIH and is now a standard in hospital and cell
biological laboratories. Further, in the mid- to late-1950s at the NIH,
she was surrounded by a rich scientific environment in what was an
exciting time in science. Marshall Nirenberg, PhD, who cracked the
genetic code while at the NIH, had an apartment in the same building
as Ruth and Al on the NIH campus. She also saw the development of
the shift in cancer treatment from the standard of surgery and radia-
tion to the newly-evolving method now known as chemotherapy, in
particular, the successful treatment of cancer with methotrexate.
After completing her residency, Ruth accepted a job with the NIH
Division of Biologics Standards (DBS). As a “free-floating” patholo-
gist, working with scientists in the NCI and the National Institute of
Arthritis and Metabolic Disease (NIAMD), Ruth sought out and
developed true collaborations where scientists worked with each other
instead of for each other. In this collaborative capacity, as with many
aspects of her career, Ruth demanded a great attention to detail. This
is illustrated by the fact that despite a strong interest in studying the
link between viruses and cancer, she refused to allow her name to be
included as an author on the now-classic paper by Sarah Stewart, MD,
PhD, and Bernice Eddy, PhD, in which they established the link
between the SV-40 virus and animal tumors. This refusal to be
included as an author on a seminal work derived from the fact that she
felt not enough attention to detail had been used in the study. This
absolute reliance on accuracy would pay off in her later work where
she developed ultimate safety in the worldwide use of the polio
vaccine.
Growing up in the mid-20th century, Ruth had firsthand knowledge
of the fear engendered in the general population by polio, a fear that
lasted until 1955 when Jonas Salk’s injectable polio vaccine was
declared safe and effective. However, soon after the release of the vac-
cine, two batches generated from the same company were tainted with
infectious virus, resulting in 40,000 illnesses, 50 cases of paralysis, and
5 deaths. It was determined that the cause was the unrealized
3Ruth L. Kirschstein—The Woman and Her Legacy
incomplete inactivation of the virus by the method being used at the
time to create a “safe” vaccine. To address this issue, the NIH devel-
oped a committee to develop new methods of inactivating the virus
and hired Ruth to perform safety testing on the resulting vaccine.
Tapping into her attention to detail, she developed the most effective
and reproducible procedure for testing the safety of the vaccine in ani-
mals. Around this time Albert Sabin, MD, developed his oral polio
vaccine that utilized an attenuated form of the virus, which had its
own potential public health problems. In response to these concerns,
the NIH developed a committee to develop standards for determining
the safety of new batches of polio vaccine. Once again, through her
hard work Ruth developed a method to test the safety of the vaccine
in a manner that was reliable and reproducible. She taught this
method to manufacturers around the world, subsequently becoming
the standard by which all produced lots of polio vaccine were tested
for safety.
As a result of the excellent work performed on vaccine safety, Ruth
was named the chief of the DBS Laboratory of Pathology in 1965, a
mere 8 years after joining the division. As the chief, she was known for
her evenhanded management of individuals and for her treating every-
one with respect. She was known as an excellent mentor, being nurtur-
ing yet allowing her trainees and employees to shine on their own. She
also recognized talent and nurtured it, especially when that talent was
present in a minority individual. Her energy and enthusiasm for sci-
ence was a major draw for all who worked for her and inspired many
to work hard and dedicate themselves to protecting public health. This
leadership style led her group to develop safety tests for other viruses
and vaccines, such as hepatitis B; work that led to future vaccine
development.
In 1972, the DBS was transferred from the NIH to the Food and
Drug Administration (FDA), where Ruth was made deputy associate
commissioner for science. Immersing herself in the position, she
learned all aspects associated with the job, including law, bureaucracy
(and how to avoid being snared in it), and administrative finesse; all
talents that would serve her well in her future career. After one and a
half years, she applied for, interviewed, and in 1974 successfully
became, the first female director of an NIH institute, the National
Institute of General Medical Sciences (NIGMS). As director, she
4 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
staffed the institute with highly qualified individuals whom she insured
for quality by interviewing personally. She developed a team who
could effectively balance smart decision-making, team play, and hard
work, thereby increasing morale at the institute, which ultimately
strengthened the NIGMS. She was involved in all aspects of the insti-
tute, almost to the point of micromanaging. However, by bringing her
great attention to detail to an administrative position, she was acutely
aware of everything that happened within all levels of the institute.
As director, Ruth understood that basic research does not necessar-
ily rely on a specific outcome but instead on the results from a growth
of knowledge through incremental advances essential for progress. Up
until her tenure as director, money for basic research and money for
research training, in the form of the NRSA, were separate entities.
However, Ruth felt that these two programs should be integrated
because the combination of training and research would ensure the
long-term support of both. Further, she developed solid relations with
politicians on Capitol Hill through her persistence and honesty. She
had an uncanny ability to describe difficult concepts in clear terms and
to relate the necessity for funding by relating health issues to the
personal lives of senators and representatives. As a result of her hard
work, the NIGMS budget quadrupled during her tenure.
Soon after starting her position as director, Ruth was asked to chair
an NIH committee whose goal was to evaluate the grant peer-review
process. She recognized that the process was prone to human error and
natural human bias and that the system needed revamping to provide
protections from both. Further, she noted the “incestuous” nature of
selecting reviewers, in which a reviewer nominated a replacement when
they rotated off. This, combined with gender and ethnic biases in the
review process resulted in a system that contained few women and few
minorities. Her year-long study developed policies that she introduced
and are still in place today, changes were made that included
members self-nominating for inclusion on a review panel, applicants
being allowed to see the critiques from their review, and allowing
applicants to argue their case if they believe an unfair or biased
critique was given.
Ruth also worked hard to diversify the NIH and examined the pro-
grams that targeted underrepresented minorities. She noted that the
diversity program at the time, Minority Access to Research Careers
5Ruth L. Kirschstein—The Woman and Her Legacy
(MARC), while being a solid program, didn’t do enough to fully
address the issues and only affected a small number of minorities at a
handful of institutions. From this realization she developed a new pro-
gram, the Honors Undergraduate Research Program (HURP), which
became a component of MARC. In this program, a series of science
honors classes and summer research programs could be implemented
at minority institutions to pique interest in science and science careers.
Within 10 years of implementing this program, 76% of the program
trainees had enrolled in graduate or professional schools. Further, and
more generally, Ruth believed that the quality of training depended on
its symbiosis with research; and that by investing in training, either to
pique an interest in minorities or to support the training of professional
school students, the future of science would be strengthened. By being
such a strong proponent of training, Ruth was honored in 2002 by
having her name added to the NIH’s main training program, which
thereafter was known as the Ruth L. Kirschstein NRSA training
grants.
In addition to fighting for women and minorities in employment
and in education, she also fought to change the way people thought
about these populations, particularly women, from a scientific and
medical standpoint. She knew that science upheld the fact that women
and men were not necessarily the same in terms of clinical responses to
treatment or even the health issues they dealt with. However, at the
time women, by law, were excluded from clinical trials. Through her
work, she became the driving force behind changing the laws about
the inclusion of women in clinical studies, raised awareness about the
importance of addressing men and women differently in terms of medi-
cal and clinical issues, and was instrumental in developing what would
become the Office of Research on Women’s Health.
As director of the NIGMS, Ruth oversaw many programs and
initiatives that are widely known today. She formed the Recombinant
DNA Risk Assessment Committee whose task was to develop guide-
lines for recombinant DNA research. The results of this committee rev-
olutionized this field producing research that resulted in several Nobel
Prizes. She oversaw the development of a database of DNA sequences,
which eventually became GenBank, and after years helped develop
this program into the National Center for Biotechnology Information
(NCBI). She also assisted in working to develop policies that became
6 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
the Human Genome Project in 1990, a project whose goal was to
sequence the entire human genome. She even became a subject in a
clinical trial for combination chemotherapy and multimodal treatment
when she was diagnosed with inflammatory breast cancer, a treatment
that saved her life and is commonly used today.
In 1993, she became the acting director of the NIH while Harold
Varmus awaited his approval by Congress. When Varmus took over
the reins, Ruth left the NIGMS to become deputy director of the NIH.
In this capacity she worked with Varmus, not always agreeing with his
opinions, but working hard to make his visions reality and to truly
transform the NIH. During this time, too, as science moved fast in
many different avenues, Ruth and Varmus worked hard to explain
science and its relevance to politicians, the media, and the population
at large. Her position as deputy director continued until 2000, when
Varmus left the NIH and Ruth once again became the acting director.
During her subsequent 2-year tenure she saw the completion of the
Human Genome Project, the development of ClinicalTrials.gov, a
database where volunteers could search for ongoing medical studies,
the establishment of the National Center on Minority Health and
Health Disparities, the creation of the National Institute of Biomedical
Imaging and Bioengineering, the establishment (in accord with a new
law by President George W. Bush) of the NIH Guidelines for
Research Using Human Embryonic Stem Cells, and supported the cre-
ation of the Biomedical Research Infrastructure Network, a program
aimed at broadening the geographic distribution of NIH funds.
Attesting to her true leadership abilities, she led the NIH through the
terrorist attacks of September 11, 2001, and oversaw the transforma-
tion of the NIH security in response to that tragedy.
After stepping down as acting director, Ruth stayed on at the NIH
serving as senior advisor to the new director, Elias Zerhouni, MD. She
continued to work at the NIH until her death at age 83 in 2009, a
death that occurred as she wished: With her family by her side, at a
place that she loved, the NIH Clinical Center. Ruth L. Kirschstein was
a truly amazing person. Her life experiences, growing up the child of
Jewish immigrants, both of whom were teachers who instilled an atti-
tude of achieving whatever you put your mind to, along with the atten-
tion to social responsibility, created a sense of justice and
determination that allowed her to persist through any challenges and
7Ruth L. Kirschstein—The Woman and Her Legacy
accomplish great things. She made an impact on everything she did as
a clinician, a scientist, and an administrator; addressing issues of public
health, health disparities, inequities in science, and creating many
aspects of the NIH that are thriving programs today. Most impor-
tantly, she was a teacher, mentor, and advisor to many. Ruth loved to
harness peoples’ passions, tapping into those passions to develop unno-
ticed talents through her mentoring and nurturing. She was a tough
mentor, but fair and caring, just like the teacher in school who pushed
you to your limits because they saw what you were capable of even
when you couldn’t see it for yourself. People were always the main
focus for her and she loved the role of teacher. Finally, she believed
strongly that providing excellent training to young scientists was the
way to ensure the future of scientific endeavors, something she worked
tirelessly to provide and ultimately creating one of her many enduring
legacies, the Ruth L. Kirschstein NRSA training grants.
1.2 THE LEGACY—THE RUTH L. KIRSCHSTEIN NRSA GRANTS
The overall goal of the NIH Ruth L. Kirschstein NRSA training
grants is “to help ensure that a diverse pool of highly trained scientists
is available in appropriate scientific disciplines to address the nation’s
biomedical, behavioral, and clinical research needs.” Full information
with links to the parent funding announcements for these awards are
available on the NIH web site “F-Kiosk—NRSA Individual
Fellowship Funding Opportunities” (
https://researchtraining.nih.gov/
programs/fellowships
). There are presently five categories of NRSA
awards, each of which addresses a different stage or type of training.
1.2.1 F30—Individual Predoctoral MD/PhD and Other Dual
Doctoral Degree Fellows
The purpose of the F30 award is to support individual predoctoral
MD/PhD and other dual degree candidates with the goal that this
training will increase the number of physician scientists in basic, trans-
lational, and clinical research. Physician scientists play an important
role in basic biomedical, translational, clinical, behavioral, epidemio-
logic, prevention, and services research.
1.2.2 F31—Individual Predoctoral Fellows
The purpose of the F31 award is to provide support for promising doc-
toral candidates who will be performing dissertation research and
8 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
training in scientific health-related fields relevant to the missions of the
participating NIH institutes. The award will provide up to 5 years of
support for training leading to the PhD or equivalent degree.
1.2.3 F31 Diversity—Individual Predoctoral Fellowships to
Promote Diversity in Health-Related Research
The purpose of the F31 Diversity award is to provide up to 5 years of
support for research training leading to the PhD or equivalent degree
or the combined MD/PhD degree. These awards differ from the F30
and F31 in that they are meant to provide opportunities for underrep-
resented minority groups, thereby enhancing the diversity of the bio-
medical fields. These groups include underrepresented racial and ethnic
minorities (including African American, Hispanic, Native American,
and United States Pacific Islanders), individuals with disabilities, and
individuals from socially, culturally, economically, or educationally
disadvantaged backgrounds.
1.2.4 F32—Individual Postdoctoral Fellowships
The purpose of the F32 award is to provide support to promising post-
doctoral applicants who have the potential to become productive and
successful independent research investigators.
1.2.5 F33—Individual Senior Fellows
The purpose of the F33 award is to provide senior fellowship support
to experienced scientists who wish to make major changes in the direc-
tion of their research careers or who wish to broaden their scientific
background by acquiring new research capabilities as independent
research investigators.
As with any NIH award, these grants are available for US citizens
only. It is important to note, too, that not all NIH institutes and
centers participate in the Ruth L. Kirschstein NRSA program. The
participating institutes are listed on the parent funding announcement
for each of the individual awards and have links that will provide
more information for special requirements that may be in place of the
different institutes.
9Ruth L. Kirschstein—The Woman and Her Legacy

CHAPTER
2
2
The People Behind the Curtain—Understanding
the Review Process
2.1 THE NATIONAL INSTITUTES OF HEALTH (NIH)
Almost everyone in academic science has heard of the NIH, as have
many people in the United States. In the world of academic research,
receiving funding from the NIH is considered the epitome of obtaining
money for many investigators and institutions. However, many trai-
nees, while having heard of the NIH, may not know how the institute
is structured or what different types of funding mechanisms are avail-
able. The NIH, located in Bethesda, Maryland, is the broad overarch-
ing conglomerate of federal research institutes responsible for
biomedical and health related research and falls under the control of
the Department of Health and Human Services (DHHS). Although
some people may mistakenly think that the NIH is a single entity, it is
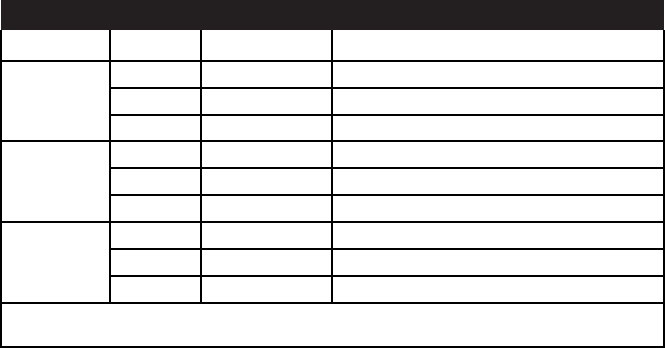
in fact made up of 27 individual institutes and centers that focus on
research related to a variety of different disciplines in biomedical
science (see
Table 2.1).
Individual institutes within the NIH accept most types of grant
applications, which can be either solicited or unsolicited. Solicited
applications are submitted only in response to a specific Request for
Application (RFA) and usually involve a more focused research area
that addresses a specific subarea of interest or research within an
institute. These RFAs many times derive from money that has been
budgeted within an institute to cover research targeting a very specific
or focused topic or disease. In contrast, the unsolicited applications
do not adhere to a specific announcement, may be submitted at any
of the cycle deadlines, and can investigate any area of research as
long as the focus of the project falls under the umbrella of a particular
institute.
A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant. DOI: https://doi.org/10.1016/B978-0-12-815336-9.00002-8
© 2018 Elsevier Inc. All rights reserved.

In addition to the Ruth L. Kirschstein training grants, which are
the focus of this book, the NIH also has multiple different mechanisms
of grant support for a variety of different purposes:
Individual Research Grants (R-series):
• R01 Research Project Grant: These grants are in many ways the “Holy
Grail” of research funding. This mechanism supports a discrete, speci-
fied project that will be performed in the lab of the named investigator
and will provide up to $250,000/year for up to 5 years.
Table 2.1 Individual Institutes and Centers Within the NIH
Institutes of the NIH
National Cancer Institute (NCI)
National Institute of Allergy and Infectious Diseases (NIAID)
National Institute of Dental and Craniofacial Research (NIDCR)
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
National Heart, Lung , and Blood Institute (NHLBI)
National Institute of Mental Health (NIMH)
National Institute of Neurological Disorders and Stroke (NINDS)
National Institute of Child Health and Human Development (NICHD)
National Institute of General Medical Sciences (NIGMS)
National Eye Institute (NEI)
National Institute of Environmental Health Sciences (NIEHS)
National Institute on Alcohol Abuse and Alcoholism (NIAAA)
National Institute on Drug Abuse (NIDA)
National Institute on Aging (NIA)
National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
National Institute of Nursing Research (NINR)
National Institute on Deafness and Other Communication Disorders (NDCD)
National Human Genome Research Institute (NIGRI)
National Institute of Biomedical Imaging and Bioengineering (NIBIB)
National Institute on Minority Health and Health Disparities (NIMHD)
Centers of the NIH
Center for Scientific Review (CSR)
National Center for Advancing Translational Sciences (NCATS)
Center for Information Technology (CIT)
John E. Fogarty International Center (FIC)
National Center for Complementary and Integrative Health (NCCIH)
National Center for Medical Rehabilitation Research (NCMR)
National Center for Research Resources (NCRR)
12 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
• R21 Exploratory/Developmental Research Grant: These grants sup-
port exploratory or developmental research by providing support
for the early and/or conceptual stages of project development. These
grants are 2 years in duration and provide up to $275,000 for the
length of the funding period.
• R03 Small Grant Program: These grants support small research pro-
jects that can be carried out in a short time period with limited
resources and provide $50,000/year for up to two years.
Program Project/Core Grants (P-series):
• P01 Research Program Project Grant: This granting mechanism pro-
vides support for an integrated, multiproject research program that
involves a number of independent investigators who share knowl-
edge and common resources.
• P30 Center Core Grant: These grants support shared resources and
facilities for categorical research by a number of investigators from
different disciplines who provide a multidisciplinary approach to a
joint research effort or from the same discipline to focus on a com-
mon research problem.
Pathway to Independence (K99/R00):
• These grants are intended to assist postdoctoral trainees in transition-
ing to their first independent research position and provide up to 5
years of support for early career investigators. These grants consist of
two parts: (1) K99 phase: 12 years of support for highly promising
postdoctoral researchers, and (2) R00 phase: Support for up to the first
3 years of the independent research career. Transition from the K99
phase to the R00 phase is not automatic and is contingent on having a
research position and programmatic review of the K99 phase.
While these are the most prominent and notable granting mechan-
isms, they are by no means the only ones. This book will focus on the
constructing of the F30, F31, F31 Diversity, and F32 Ruth Kirschstein
training grants.
2.2 INDIVIDUALS INVOLVED IN GRANT MANAGEMENT
Although many individuals are involved with the construction, submis-
sion, review, and management of a grant, there are two key individuals
13The People Behind the Curtain—Understanding the Review Process
within the NIH who are involved with the direct management of the
review process and the post-award project:
• Scientific Review Officer (SRO): The SRO is the person who
manages the review process for grant applications. They are respon-
sible for analyzing each submission for completeness, recruiting
reviewers, managing conflicts of interest, assigning applications for
review, attending the review panel (also known as a study section),
orienting the members to the operations and policies of the study
section, documenting a summary of discussions and recommenda-
tions, and preparing the summary statements for the applicants.
• Program Officer (PO): The PO manages and advises projects (pre-
and post-award) as they relate to the individual institutes’ research
focus. They are responsible for advising applicants about the
appropriateness of a project to an institute’s research focus or a
specific RFA, making funding recommendations to the institute
based on the reviews, overseeing the progress of the funded projects,
encouraging and developing new scientific opportunities for applica-
tions, and helping develop NIH policy.
2.3 THE REVIEW PROCESS
The process by which NIH applications are reviewed is probably one
of the most confusing, unclear, and even sometimes infuriating aspects
of the federal granting system for investigators who have submitted
their work for consideration. In fact, sometimes trying to get funded,
or even receiving a favorable review, can feel like you are trying to hit
a moving target, blindfolded, with a gun that does not shoot straight!
This fact is particularly true for predoctoral or postdoctoral trainees
who are at the beginning of their scientific career and most likely have
never written a grant before, let alone experienced receiving reviews
for submitted grants, or had the experience of serving on an NIH study
section. In fact, many times faculty members themselves who serve as
their mentors do not fully understand the review process until they
have served on a study section and experienced firsthand the discus-
sions, biases, and in some cases outright prejudices that exist as deci-
sions on the quality of a grant are being made. An understanding of
how the review process works, the natural human bias and prejudices
that come into play, and the circumstances under which individual
reviewers may be evaluating the applications, can provide invaluable
14 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
information to a trainee to assist them in writing the best application
that they possibly can.
One of the first things essential for an applicant to understand is
that unlike the R-series of research grants (R01, R21, and R03), in
which the applications are sent to and reviewed by a study section con-
vened by a specific institute (e.g., the NCI or the NIGMS), the Ruth
L. Kirschstein NRSA training grants are, in the majority of cases,
reviewed by an interdisciplinary group. There are 20 different interdis-
ciplinary study sections that consist of recurring special emphasis
panels with each of these study sections having a different scientific
focus (For a complete list see https://public.csr.nih.gov/StudySections/
Fellowship/Pages/default.aspx). The members of these study sections
are ad hoc, meaning they do not hold permanent reviewer status on
this study section, and are recruited based on their scientific expertise
and how well that expertise fits within the scientific focus of each indi-
vidual group. For example, the F08 study section on Genes, Genomes
and Genetics reviews those applications that focus on the genetics,
genomics, and gene regulation of prokaryotic and eukaryotic systems,
while the F07 study section on Immunology reviews applications that
are aimed at understanding the role of the immune system in all its
different interactions and responses. Because the review is interdisci-
plinary and not dependent on the institute that will ultimately fund the
grant, these members review grants whose research coincides with the
scientific focus of each particular study section, with the final funding
decisions being made by the appropriate NIH institute.
Within each interdisciplinary study section, the members will evalu-
ate and discuss all five types of F-series training grants (F30, F31, F31
Diversity, F32, and F33). Therefore, each reviewer must be aware of
the nature of the training grant they are reviewing (i.e., predoctoral
training grant vs. postdoctoral training grant) and evaluate the applica-
tion accordingly. During study section the applications are separated
into the five different fellowship types with each group being discussed
as a unit. This means that the F30 applications are usually discussed
first, followed by the F31 Diversity, the F31, the F32, and if any have
been submitted, the F33 applications. Depending on the number of
applications under consideration, all of the grants within each group
may be discussed or only 50% of the grants will be discussed. If, for
example, only six F30 applications are under consideration, then all of
15The People Behind the Curtain—Understanding the Review Process
these grants will be discussed, regardless of the initial impact score,
and therefore receive a score. However, in the case of the F32 or F31
applications, which are by far the largest in number and can sometimes
have upward of 6070 applications each, only half will be discussed.
The cutoff for which applications will be discussed varies from study
section to study section, but usually falls at about the 50% mark. If a
grant application is not discussed in study section, it will receive a des-
ignation of “Not Discussed” on the final Summary Statement in lieu
of a score.
The review process begins with the recruitment of the members for
each interdisciplinary study section. The SRO invites approximately
2530 reviewers for each study section. These reviewers are chosen
based on their scientific expertise, which is determined by how well
their research focus fits into the scientific topic for that interdisciplin-
ary section. Selecting members based on their research focus provides
a panel of investigators who will be well versed in the general topic
being reviewed and should therefore be able to provide informed
reviews and solid criticisms of each grant. Reviewers are ad hoc, mean-
ing that no one person is given a permanent appointment to this
review group and must receive an invitation for each new study sec-
tion. However, people can be invited back repeatedly to serve on a
study section. A repeat presence is determined by the SRO and can be
dependent on the scientific expertise of the reviewer, the quality of the
reviews that the person provides, the contributions that the reviewer
makes to discussions, or all of the above.
Once the study section roster is established, conflicts of interest
between a study section member and any aspect of the submitted
grants must be determined. The SRO will provide all members with a
list of the grants to be considered. Each member examines this list to
see the name of the applicant, the name of the sponsor, the name of
the cosponsor (if appropriate), the names of the people who wrote the
letters of recommendation, and at what institute the work is being per-
formed. If a study section member knows the applicant, sponsor,
cosponsor, and in some cases the people who wrote the letters of rec-
ommendation personally, has worked with any of them in the past, or
works at the same institute from which the application derived, that
member is considered to be in conflict with the application. The
reviewer is then recused from reviewing that application and when that
16 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
grant is being discussed physically will leave the room so as to not
hear the discussion associated with the application.
Once the roster is established and conflicts of interest are deter-
mined, the SRO assigns up to 12 grants to each study section member.
These assignments are made based on the known scientific expertise of
each reviewer with the SRO making all best attempts at providing the
best fit between application and reviewer. However, it is important to
note, particularly as you write your grant, that frequently, if not
always, someone will be reading your grant that is not an expert in
your field of research. The person reading your grant may be familiar
with the techniques and theories you put forward, but will not have
firsthand experience with the exact field in which you are proposing
for your research. This means they will be versed enough to critique
your logic, evaluate your preliminary data, comment on the validity of
the proposed experiments, and agree or disagree with conclusions that
you draw. However, they will most likely NOT implicitly understand
the details of the field. Therefore, it is important to make the assump-
tion that the person reading your grant knows nothing about the field
in which you are writing so that you can write clearly and explicitly. If
you do not make this assumption, and write your science so only an
expert in the field would understand it, you will receive a lower score
than might be expected.
Once the applications are assigned, the reviewers will be designated
as Reviewer 1, Reviewer 2, or Reviewer 3. Reviewers 1 and 2 are
responsible for providing detailed descriptions of the strengths and
weaknesses of each individual review criterion (see below) along with a
description of the overall impact and merit of the grant. In contrast,
Reviewer 3 is only required to provide a description of the overall
impact, although they are strongly encouraged to provide detailed
information on all criteria. Further, the reviewers will have a mix of
grants (F30, F31 Diversity, F31, F32, or F33) and a mix of new sub-
missions versus resubmissions. It is the responsibility of the reviewer to
recognize the type of grant they are reading, be aware if it is a new
submission or a resubmission, and be aware of what their reviewer sta-
tus is, because slightly different criteria are used to evaluate the differ-
ent types of grants with more extensive information being required
based on the reviewer status.
17The People Behind the Curtain—Understanding the Review Process
After receiving their assignments the reviewers are given approxi-
mately 34 weeks to read, review, and critique each of the grants for
which they are responsible. While reading the application, the
reviewers are asked to evaluate five different primary criteria to arrive
at an initial impact score: (1) Fellowship applicant; (2) Sponsors,
Collaborators, and Consultants; (3) Research Training Plan; (4)
Training Potential; and (5) Environment. Each of these criteria is eval-
uated on a score of 19 according to the definitions supplied to each
reviewer by the NIH (
Table 2.2).
In addition, if applicable, human subjects, vertebrate animals, and/
or biohazards are also incorporated into the overall impact score. If
the application is a resubmission, the reviewers are asked to determine
how well the applicant addressed the previous comments, which is also
a contributing factor to the overall impact score. All of these latter
items, while not receiving scores individually, are taken into consider-
ation when determining the overall impact score. Finally, the reviewers
examine such nonscored items as Responsible Conduct of Research,
Applications from Foreign Organizations, Research Sharing Plan,
Select Agents, and appropriateness of the requested budget.
While reading the grant, each reviewer is responsible for writing a
critique addressing each of the five main criteria listed above. These
critiques are intended to be detailed and to discuss in a constructive
manner the strengths and weaknesses of each of these criteria. In addi-
tion, the reviewers provide an overall impact statement: A paragraph
Table 2.2 NIH Scoring Table and Descriptors
Impact Score Descriptor Guidance on Strengths/Weaknesses
High 1 Exceptional Exceptionally strong with essentially no weakness
2 Outstanding Extremely strong with negligible weaknesses
3 Excellent Very strong with only some minor weaknesses
Medium 4 Very good Strong but with numerous minor weaknesses
5 Good Strong but with at least one moderate weakness
6 Satisfactory Some strengths but also some moderate weaknesses
Low 7 Fair Some strengths but with at least one major weakness
8 Marginal A few strengths and a few major weaknesses
9 Poor Very few strengths and numerous major weaknesses
Minor weakness: An easily addressable weakness that does not substantially lessen impact. Moderate
weakness: A weakness that lessens impact. Major weakness: A weakness that severely limits impact.
18 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
in which they describe their opinion on the overall impact of the appli-
cation, and provide descriptions of what they perceived to be the main
score driving issues. It is important to note that the overall impact
score is NOT an average of each individual criterion score but instead
reflects how each reviewer believes the individual strengths and weak-
nesses of the application will contribute to provide an overall training
experience for the applicant. Therefore, it is possible to have an overall
impact score that is either higher (worse) or lower (better) than the
sum of its parts.
After the critiques have been submitted the SRO calculates the
average of the overall impact scores of the three initial reviewers to
determine an initial impact score. This initial impact score is used to
establish the discussion order for the study section meeting. On the day
of the meeting the reviewers receive a sheet with a list of all of the
grants listed in the order of discussion. On this list the grants are
broken down into the different type of grants (F30, F31 Diversity,
F31, F32, or F33) and within each type the grants are ranked from
best (lowest score) to worst (highest score) based on their average ini-
tial impact score. In addition, these scores help determine which grants
will or will not be discussed. For example, as stated above, approxi-
mately 50% of the F32 grants are discussed. This means that if 60 F32
grants were submitted, approximately 30 will be discussed.
At the study section meeting, which can occur all in 1 day or be
extended to 2 days depending on the number of applications under
consideration, the discussion proceeds in the order predetermined by
the averaged initial impact scores and follows the same format for
each grant being discussed. For each application the chair of the study
section will announce if any of the study section members are in con-
flict, and if so, they will be asked to leave the room for the duration of
the discussion. The chair then announces the name of the applicant,
the title of the application, and the institute at which the work will be
performed. The reviewers are asked to state their overall impact score
for the application after which Reviewer 1 provides a brief description
of the application, the strengths and weaknesses of each section, and
what issues drove their score. Reviewer 2 then adds to the discussion
by providing their opinion of strengths and weaknesses and score driv-
ing issues. Reviewer 3 also does the same. For Reviewers 2 and 3 it is
acceptable for them to state “nothing to add ” should their opinions of
the application not differ significantly from Reviewer 1. The floor is
19The People Behind the Curtain—Understanding the Review Process
then opened for discussion where all the members of the study section
are given the opportunity to ask questions of the reviewers for clarifi-
cation of the issues, to ask how heavily the issues contributed to the
score, why certain issues weighed more heavily than others, why one
reviewer gave a significantly different score from another, etc.
In the days before the Internet, only the three assigned reviewers
would have access to the full grant application. The remaining study
section members would be provided with the Specific Aims page only.
Therefore, decisions were heavily dependent on the quality of the three
reviewers’ critiques and presentations. However, in today’s electronic
age, all members of the study section have easy access to every single
grant application being discussed. Although discussions are still depen-
dent on the quality of the critiques and presentations by the three
reviewers (since they are the only ones who have had time to thor-
oughly read the full application), any member can easily access the
application to verify facts or to read individual sections to draw their
own conclusions relating to score driving issues or check and validate
facts under discussion.
At the conclusion of the discussion the chair will then ask the
reviewers to state their new overall impact score, which may or may
not have changed as a result of the discussion. These new impact
scores establish a range within which all members of the study section
should vote. For example, if the new impact scores are 3, 4, and 5, the
range in which all study section members are encouraged to vote is a
35. However, based on the discussion, each individual member may
choose to vote outside of this range if they feel it is appropriate. After
the range is established, the chair will then ask if anyone will be voting
outside of the range and all members doing so must indicate that they
will be doing so by raising their hand. They are not required to state if
they are voting higher (worse) or lower (better), simply that they are
voting outside of the range. All study section members enter their
scores online and the final score that is sent to the applicant is the
average of the scores for all study section members.
This entire process, from the initial introduction of the application to
the final entering of scores can take anywhere between 5 min and 15 min
depending on the level of agreement that exists between the reviewers,
how many questions are asked by other study section members, and the
extent of discussion that is required to arrive at a consensus.
20 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
It is important to note that the sole function of the study section is
to determine a score for each individual application. This score is
derived from the strengths and weaknesses of each application and as
such reviewers are not allowed to draw comparisons between applica-
tions. The reviewers are not allowed to discuss the fundability of the
application because their job is to simply weigh the merits of each
grant. Funding is then determined by each individual NIH institute
that ultimately provides the funding. Each individual institute has dif-
ferent criteria and a different budget for the level of fundability and it
is this budget and criteria that determines the pay line cutoff.
Therefore, an application with a score of a 29 may be funded
through the National Institute on Aging. However, a different grant,
also with a score of 29, may not be funded through the National
Cancer Institute.
2.4 THE ROLE OF HUMAN NATURE IN THE REVIEW PROCESS
The previous discussion provided a description of the process by
which applications are reviewed. In a perfect world, all things would
be equal and all applications wou ld be judged by equal standards.
However, the reviewers are human and as such basic human nature,
and all of the biases, prejudices, and influences (both consci ous and
unconscious) that go along with human nature, come into play in the
review process. One of the influences that can affect the quality of
the review given by a member is simple time management. It is essen-
tial to remember that serving on a study section is not the sole job,
nor the primary job, of the rev iewer. T hey are busy faculty members
who are juggling many differe nt roles, including running a lab, men-
toring train ees, teaching, and admini strative duties. Although they
are prov ided sufficie nt time to adequately evaluate the ir grants, many
times they will wait until the last minute due to simple procra stina-
tion. It is also more likely that due to busy schedules they may not
have a choice but to read them at the last minute . They will then be
confronted with a sho rt period of time in which to rea d and evaluate
up to 12 grants, each of which averages 50 pages and can take any-
where between 2 h and 4 h to complete. Further, the conditions
under which a reviewer may actually be readin g your application
may not necessarily be optimal. Sitting on study section is not the
reviewer’s “day job,” but something they are paid to do as a service
to the NIH, and it is a function that is extracurricular to their
21The People Behind the Curtain—Understanding the Review Process
normal responsibilities. Therefor e, many times reading is done under
what Peg AtKisson formerly of the Grant Writers’ Seminars and
Worksho ps described as the “2-2-2 Rule.”
Imagine you are a reviewer who runs a lab with seven workers.
Among these workers are two graduate students who require a lot of
time because they are new and inexperienced. You spend a majority of
your day with these students, more time than you expected, making
you frazzled because you didn’t accomplish the work you needed to
get done. You get home from work around 6:30 in the evening to your
two young children. They’ve missed you and you want to spend time
with them. They need to be fed, and then they don’t want to go to bed
when they are supposed to. Finally, you get them to bed and they are
asleep. Because your nerves are shot you decide to have two glasses of
wine to calm down. Once the wine is consumed and your nerves have
settled, you sit down to start reviewing your list of grants, probably
around 10:30 at night. Obviously these conditions are not necessarily
conducive to a reviewer’s clarity of thinking. However, although
slightly exaggerated, this scenario is not too far off the mark and pro-
vides an illustration for the potential state of mind in which a reviewer
will evaluate a grant. Under these conditions a person may not neces-
sarily be capable of focusing his/her mind to provide an unbiased eval-
uation of an application.
Along these lines, a tired reviewer will naturally fall back on their
natural inclinations while they evaluate an application. For example,
in a Ruth L. Kirschstein training application, the evaluation of the
Research Training Plan (the science portion) is meant to focus less on
the nature of the science and more on how the proposed scientific plan
will contribute to the overall training. However, we as scientists are
trained to think about how to develop a logical argument to support a
hypothesis, how to provide solid preliminary data to support feasibil-
ity, how to describe solid experimental design to address the hypothe-
sis, and to discuss how our results will impact the field. While all of
these things contribute to and are indicative of a solid training experi-
ence, the nature of reviewers is to focus on these issues for the science
itself and not for how the science will provide a solid training.
Although this is a very subtle difference in perspective, it can make a
significant difference in the evaluation of a training application and
how that application will fare in study section.
22 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
Another aspect of human nature that greatly affects the review is
the fact that people simply have differences in opinion. These differ-
ences in opinion can, and do, affect all of the individual criteria under
evaluation. Also, because of these differences in opinion, and the
biases, personal experiences, and prejudices that contribute to the for-
mation of these opinions, there will be very different viewpoints on
what will constitute a solid training potential. Several of these differ-
ences in opinion manifest themselves in the following ways.
2.4.1 The Bias of the “Big Name” Sponsor
Investigators who are leaders in their fields have achieved this status
through years of hard work and a history of publishing groundbreak-
ing science in highly respected journals. This status usually means that
the “ big name” sponsor has a reputation that precedes them. Because
they are leaders in the field the basic assumption is sometimes made
that simply because they have this reputation that every single person
trained in their lab is going to get a top-notch training experience.
Frequently a comment such as “if they survive that lab of course
they’re going to get a good training” or “I was blinded by the Nobel
Prize” or even “they have a history of developing new techniques so of
course this novel technique (with no data to support its feasibility) will
be successful” are heard in study section. As such, a generic or brief
and uninformative training plan may be tolerated more readily from a
“big-named” sponsor, while a similar training plan from a less presti-
gious or less established sponsor would be critiqued more stringently
or harshly. Another way in which this bias may manifest itself is
through the fact that the feasibility of an extremely high-risk Research
Training Plan may be accepted more readily without preliminary data
to support it from the lab of a “big-named” sponsor. Many people on
the study section realize, however, that just because a person is an
exceptional scientist does not mean they will be an exceptional mentor
nor that high-risk science is any more technically feasible in the lab of
a “big name” sponsor.
2.4.2 The Bias of the “High-Power Institution”
As with the “big name” sponsor, an application that comes from a
“high-power institute” may be given leeway that would not be given to
many other applicants. As with the big name sponsors, high-power
institutes, such as Johns Hopkins University, Harvard University, the
Mayo Clinic, or St. Jude Children’s Research Hospital, achieved their
23The People Behind the Curtain—Understanding the Review Process
reputation through decades of producing top-notch groundbreaking
research, being the home of multiple Nobel laureates, or being places
that revolutionized education and/or medical technology. Just as
described above, descriptions of facilities and environment that are
brief and uninformative are tolerated more in applications from high-
power institutes. Further, high-risk science and the development of
novel techniques are also considered more feasible in the absence of
preliminary data than similar quality applications from non-high-
powered institutes. However untrue this reality may be, this bias still
exists and requires greater attention to detail in the preparation of the
application for investigators not in big name labs or at high-power
institutes.
2.4.3 Quantity Versus Quality—What Constitutes Solid
Applicant Productivity?
Another issue frequently debated during study section is what is con-
sidered good productivity for the applicant. This issue may be difficult
to determine and is dependent on the type of application being dis-
cussed. An applicant for the predoctoral F31 training fellowship who
is in their second year of training is not expected to have as many pub-
lications as an F31 applicant that is in their fourth year of training.
Similarly, a postdoctoral trainee who has just started their fellowship is
not expected to have publications from that lab. However, they are
expected to have a solid publication record from their graduate work.
Regardless, in both cases the issue frequently under discussion is a
comparison of the number of publications that an applicant has versus
the quality of those publications, which is determined through what I
call “the dreaded impact factor.”
A journal’s impact factor is determined by the average number of
citations a journal receives relative to the overall number of papers
published in the previous 2-year time period. While a useful rubric, it
may not adequately represent the status of a more specialized high-
quality journal for a particular field. For example, the journal
Biochemistry is considered to be the top journal in which to publish for
someone working in the field of biochemical research. However,
because it is a journal for a more specialized field the number of cita-
tions that journal receives would not necessarily be high thereby giving
this journal a lower impact factor. Therefore, in study section a publi-
cation in Biochemistry would not be considered as reputable as a
24 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
publication in EMBO Journal, a journal with a wider scope and read-
ership interest. In these situations, the bias usually exists that between
two applicants in which all else is equal; the applicant with three publi-
cations in lower impact journals may not necessarily seen to be as pro-
ductive (nor creating as much of an impact) as an applicant with one
publication in Cell, Science,orNature.
2.4.4 Quantity Versus Quality—What Constitutes a Good
Sponsor Training History?
In evaluating the sponsor, reviewers consider their history of training
mentees. The sponsor’s training history is illustrated through the num-
ber of trainees that have graduated from their lab and the positions
these trainees subsequently secured upon leaving that lab. In some
cases this evaluation is simple. For example, a junior faculty member
who has run an independent lab only for a few years and has yet to
graduate any students or complete the training of any postdoctoral
researchers will not be considered to have an adequate training history.
In contrast, a faculty member who has run their lab for 20 years grad-
uated over 20 students who secured postdoctoral positions in excellent
labs with subsequent academic faculty positions will be considered to
have an exceptional training history.
The difficulties arise when reviewers are confronted with a junior
faculty member who has successfully trained a small number of stu-
dents; however, these students have all proceeded to high-quality posi-
tions. Any investigator who has trained multiple students knows
firsthand that although you think you knew how to mentor a student
when you were just starting out, there are simply aspects of mentoring
that can only be learned through experience. However, isn’t it also true
that a junior faculty member who has trained a few students to excep-
tional positions has proven their mentoring capabilities? To some
reviewers all they see are the numbers. To other reviewers all they see
is where the students have gone, but they may consider these excep-
tional placements as an aspect of the student’s excellence and not nec-
essarily an indication of the mentor’s capability. Yet some reviewers
are capable of realizing that part of the excellence of where the student
has gone must certainly derive from the mentoring capacity of the
sponsor. Again, these differing considerations result from differences in
opinion between what each reviewer believes to be fact, opinions that
derive from their individual career experiences.
25The People Behind the Curtain—Understanding the Review Process
2.4.5 The Bias of a Large Laboratory Versus a Small Laboratory
Environment
One important aspect to be considered when evaluating the training
environment is the number of individuals that will be working within
the laboratory during the training period. One applicant may be work-
ing in a laboratory that only has two students and a laboratory techni-
cian or manager while another applicant may be working in a
laboratory with seven students, five postdoctoral researchers, and three
laboratory technicians. While each of these environments have their
strengths and weaknesses, the reviewers many times view these
strengths and weaknesses differently. While a small laboratory environ-
ment will allow the sponsor to have more hands-on training with the
applicant, the small environment may be seen to be not as conducive
to interactions that provide additional training experiences. In con-
trast, a large laboratory environment provides multiple opportunities
for the applicant to interact with a variety of people at very different
stages in development. However, in that large environment the ability
of the sponsor to provide a significant amount of hands-on training
with each individual person is significantly called into question. The
question then boils down to the issue of what is more important in the
training, the access to multiple “ mentors” (a large laboratory environ-
ment) or the ability of a single mentor to truly shape and mold an
individual (a small laboratory environment). As with anything else, the
answer to this question is different for each reviewer.
2.4.6 All Strengths and/or Weaknesses Are Not the Same—Impact
on Training Potential
Probably the single criterion that is the most subjective in the review
process is the training potential of an application. This criterion takes
into consideration all of the different aspects of the grant (including
the applicant, the sponsor, the science, and the environment) and as
such is influenced by all of the biases just described. Because of natural
differences in opinion, the extents to which an individual’s strengths or
weaknesses will contribute to or detract from the training potential are
also considered differently. Further, the extent to which a particular
strength alleviates the concern of a particular weakness, and vice versa,
is also considered differently and may not necessarily be the same for
all applications under consideration. Let’s examine the situation where
an applicant may propose the development of a novel investigative
model in their Research Training Plan that is by no means certain of
26 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
success. Despite the high risk of this model, there is no preliminary
data to support the feasibility of this model. Further, all of the experi-
ments in the proposal depend on the success of this model. Assume
that this uncertain model was proposed by an applicant who is being
trained in a small lab from a junior investigator at a mid-range aca-
demic institution. In this case the Research Training Plan will receive a
low score, which will subsequently have a large impact on the overall
success of the application. In contrast, should this model be proposed
by an applicant in a larger lab from a leader in the field at a high-
power institution, the question of feasibility of this uncertain model
may not even be considered. The Research Training Plan will then
receive a good score and will not significantly affect the overall train-
ing potential. This example is only one of many that exists, not only
for the Research Training Plan, but also for all aspects of the applica-
tion, and illustrates the importance of natural bias in the review
process.
2.4.7 What Constitutes a Major/Minor/Negligible Weakness?
As described above, each of the individual criteria is scored on a scale
of 19 with each number being characterized by the quantity and
nature of weaknesses present in that section. Although the reviewers
are provided with this scoring rubric, and explicitly requested by the
SRO to hold to this rubric, the nature of a particular weakness is not
necessarily evaluated the same by all reviewers. What one reviewer
may consider being a negligible weakness, another reviewer may con-
sider being a minor or major weakness. These considerations would
then change a score from a 2 (extremely strong with negligible weak-
nesses) to a 4 (strong but with numerous minor weaknesses) or even a
7 (some strengths but with at least one major weakness). Further com-
plicating this issue is the fact that multiple aspects of an application
may contribute to or alleviate the nature of a weakness. In the example
above, the presence of the applicant in the lab of a big-named
researcher at a high-power institute could alleviate the concern of an
unproven model system from a major weakness (giving it a score of 7
or worse) to a minor or possibly even negligible weakness (giving it a
score of 4 or better).
The issue over what is considered a minor, moderate, or major
weakness is exacerbated even more when determining the score for the
overall impact of an application. As stated above, the overall impact
27The People Behind the Curtain—Understanding the Review Process
score is the opinion of reviewers of how the individual criterion, with
their respective strengths and weaknesses, will provide an overall suc-
cessful training for the applicant. While the combinations of strengths
and weaknesses within that section can influence the score of each cri-
terion, the overall impact score is then influenced by how each of these
individual sections fit together to provide a description of a solid train-
ing potential. In the context of the larger application, a weakness that
may have impacted an individual criterion heavily (an untested model
system) may not have as much importance in the overall training
potential when considered beside the other criterion (sponsor, institute,
and applicant). The importance of these strengths and weaknesses are
determined by each individual reviewer through the lens of human
nature influenced by their own personal experiences and opinions.
2.5 TIMING OF SUBMISSION IS EVERYTHING
There are three submission cycles for NIH grant applications, with
each different grant mechanism (i.e., R-series, P-series, F-series, etc.)
usually having different timelines for submission, review, financial rec-
ommendation, and start date. Within each cycle are key dates includ-
ing the due date (the absolute deadline for submission), review (when
study sections will meet to review the applications), advisory council
(the second round of review to recommend funding of scored applica-
tions), and start date (when the funding will be made available for use
by the investigator). As can be seen in
Table 2.3 78 months elapse
between the date that the application is officially submitted and
the date that a successful grant would receive the funding for the train-
ing period.
Because of the length of the elapsed time between the official sub-
mission of the application and the receipt of funds for a successful
grant, trainees have a window in which they may submit and/or resub-
mit their applications. For example, consider the case where a gradu-
ate student at the end of their second year of training will submit an
F31 grant application for the August 8 deadline. This application will
then be reviewed in October or November in what would be their third
year of training. Assuming they receive a fundable score, decisions
made on funding would be finalized in January with funds being
received by the trainee in April of their third year. In this case, the
trainee would have over 2 years of training remaining during the
28 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
funding period. Even if this application does not receive a fundable
score in the first attempt, a driven trainee and sponsor will try to
resubmit a revised application for the December 8 deadline. In this
case, assuming a successful revision was submitted, the funds would be
received in July between their third and fourth year, still providing
them with at least 2 years in their training.
Now consider a situation in which a trainee is between their third
and fourth years of training wanting to submit an application for the
August 8 deadline. Using the timeline above, if successful, the trainee
will receive the funds in April of their fourth year of training. If a
resubmission of a revised application is required, and the resubmission
is successful, the funds would not be received until July between their
fourth and fifth years of training. In both of these situations, the
reviewers will consider the dates by which the funds would be received,
compare them to the time remaining in the training process, and most
likely decide that the applicant should be nearing the end of their
training and no longer require funding to complete this process.
As with all aspects of the review process and how human nature
influences the review, different reviewers will have different realms of
experience with how long a training period should last, since different
fields may have different lengths of time for training based on the dif-
ferent types of experiments that are being utilized (e.g., research utiliz-
ing animal models requires more time than research using a defined
in vitro molecular system). Regardless, trainees and sponsors must be
attentive to where the applicant is in their training process and the
time that will elapse between submission of the application and receipt
of funds if the submission was successful.
What the discussion in this chapter highlights is that although there
are distinct guidelines, rules, and examples by which each and every
grant is expected to be reviewed with an unbiased eye, human nature
exists and as such natural bias, and sometimes even downright
Table 2.3 Submission Deadlines for the Ruth L. Kirschstein Training Grants
Due Date Review Advisory Council Start Date
April 8 June/July October December
August 8 October/November January April
December 8 February/March May July
29The People Behind the Curtain—Understanding the Review Process
prejudice, will be introduced into the review process. It is the necessity
of the applicant and the sponsor to have a basic understanding that,
although not necessarily fair or correct, these biases exist. As such, the
applicant and sponsor must critically evaluate their individual situa-
tions, make the best attempt at determining how their individual
situation may be perceived by a reviewer, and then use this knowledge
to construct the best possible grant to sell themselves and their scien-
tific ideas to the reviewers. In essence, be the salesperson that knows
their target audience and write to convince that audience.
30 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant

CHAPTER
3
3
Who Are You?—The Fellowship Applicant
The Fellowship Applicant section is one of the five major criteria the
reviewers use to evaluate the Ruth L. Kirschstein training grant appli-
cations. Within this section, there are multiple components that, when
considered together, provide the reviewers with an overall impression
of the applicant, their goals, and their qualifications. These compo-
nents include the applicant’s previous academic record and scientific
productivity, their previous research experience, their goals for the fel-
lowship training and future career, a description of why the applicant
selected their sponsor, department and institute, and letters of recom-
mendation. Each of these sections examines a different aspect of the
applicant and when taken together presents an overall picture of their
attributes and abilities. Further, several of these components provide
information that will allow the reviewers to determine whether the
training described in the application is truly a departure from previous
training experiences. Therefore, to fully sell yourself as an exceptional
applicant, regardless of your history and present circumstances, it is
important that you understand the function and importance of each
component and how each of these components contributes to the over-
all reviewer evaluation of the applicant.
3.1 THE BIOSKETCH (5 PAGES MAXIMUM)
In the Biosketch, the applicant provides information describing their
previous academic record and their scientific productivity. In addition,
they provide information, in the form of a Personal Statement, that
details how the training described in the application will provide an
overall program that is perfect for them as it relates to their individual
future career goals and ambitions and their previous training history.
The Biosketch is a standard NIH form containing explicit headings
detailing the information required, among which are the applicant’s
name, their eRA Commons name (a “username” provided by the NIH
for each individual), educational history, previous positions held, and
A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant. DOI: https://doi.org/10.1016/B978-0-12-815336-9.00003-X
© 2018 Elsevier Inc. All rights reserved.
academic and professional honors. However, unlike a Biosketch writ-
ten for an R-series research grant, which focuses more on the science,
the Biosketch for training grants focuses on training and the qualities
of the applicant. Therefore, the NIH Biosketch for a Ruth L.
Kirschstein training grant includes a section for the applicant’s aca-
demic record (i.e., previous grades and test scores). Further, other sec-
tions, such as the Personal Statement, are addressed with a focus on
the training that will be provided by the overall application instead of
describing their qualifications to carry out the proposed research.
3.1.1 Personal Statement
The Personal Statement is the section of the Biosketch in which the
applicant provides a description of their goals as a scientist, their previ-
ous research experience, and exactly how the plan described in the
application will provide them with the best possible training to
advance their career. The Personal Statement can be difficult to write,
because it is not always entirely clear exactly what information needs
to be included. However, it can be viewed, in some respects, as an
“abstract” for the entire application. Just as an abstract in a manu-
script provides the reader with a summary of research being presented
in a paper, the Personal Statement provides a summary of all of the
individual components that describe you, the applicant and how the
different aspects of the application fit together to form the perfect
training environment for you as an individual. Therefore, it is impor-
tant to include explicit statements describing the goals for your career,
your research training up to this point, how this previous training
directed you along your present career trajectory, why you selected the
mentor(s) (sponsor and cosponsor) you did, how this sponsor and
cosponsor will provide you with the training you need to advance your
career, how the present environment will enhance your training, and
how the educational program at the institute and department will give
you the training that fits your personal needs.
It is usually good to begin the Personal Statement with a solid
description of your long-term goals: “My long-term research interests
involve investigating molecular pathways that contribute to the devel-
opment of human disease with the goal of establishing an independent
research laboratory at an academic institution.” It is important that
this statement be direct and detailed but yet general enough so as not
to be viewed as disingenuous. The reviewers like to see that you have
32 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
knowledge of your general research interests (e.g., molecular pathways
that contribute to human disease) without unrealistically limiting the
potential for changing interests as you progress through your career.
Follow this statement with a description of how you became interested
in research, once again being explicit but not disingenuous or ingratiat-
ing. For example, personal statements may sometimes include state-
ments such as the following: “Ever since I was a child and I saw the
wonders of nature around me, I knew that I wanted to become a scien-
tist.” Although for some people this statement may be true, reviewers
will most likely perceive this statement as saccharine and ingratiating.
Instead, if there was a personal experience that motivated your career
goals, state it explicitly being sure that it is stated factually, but yet
with meaning: “While in graduate school, a friend was diagnosed with
and succumbed to leukemia. Upon reading about this disease, I found
that many forms of leukemia have a defining genetic characteristic of a
chromosomal translocation, which produce oncogenic fusion proteins.
From that point on I became interested in cancer biology research, in
particular cancers that derive from chromosomal translocations.”
For some applicants, it may not have necessarily been a defining
moment in their personal lives that motivated their career decisions but
instead their present path is a culmination of an overall educational
process. Regardless of whether the present training trajectory derived
from a single moment (as described above) or an overall process, provide
a brief description of your academic history. Where did you perform
your previous training (undergraduate and/or graduate if appropriate)
and how did that experience contribute to forming your present career
trajectory. If there was a specific class or teacher that made an impact on
your decision to pursue academic science or a particular research focus,
you should include that information, but in a reserved and factual
manner. It is also important to include a statement that describes the
factors driving your decision to choose your present training program
and mentor. For example, if you are a graduate student that underwent
laboratory rotations, state: “As part of the graduate program at X, I
underwent a series of laboratory rotations during my first year of graduate
school. Through these rotations, Dr. Y impressed me with her hands-on,
nurturing mentoring approach. Further, her research sparked my interest
as it relates to my long-term goals.” Finally, concisely summarize how the
overall training that you will receive is perfect for you: “Taken together,
the skills learned from my experience at this institute and completion of
33Who Are You?—The Fellowship Applicant
the training plan put forth by Dr. Y and the Departmental program, along
with ample mentoring opportunities available here, will equip me with the
diverse qualities needed to pursue a career as an independent researcher in
an academic environment.”
Sometimes an applicant may have had issues in their past or present
personal situations that may limit or affect the perception reviewers
have of the overall quality of the described training plan. These issues
may include personal family situations that require an applicant to
remain within a specific region or even the same institute to continue
their training; personal health issues that caused an applicant to take a
hiatus from their training or created a perceived gap in their publica-
tion or training history; health or personal issues of a previous mentor
that delayed publication of previous work; or even simple immaturity
and indecision at an earlier stage of training that resulted in poor aca-
demic grades. Regardless of the reason, it is important that these issues
be addressed directly, but in a professional manner, in the Personal
Statement. Remember, although it may not necessarily seem like it
when reading their critiques, the reviewers are human and understand
that even though an applicant may have the best intentions, that some-
times life simply gets in the way. Further, it is even more important to
remember that reviewers usually read applications very closely and will
see inconsistencies in your past (such as a gap in your training). If no
reason is given to explain these inconsistencies, the reviewer will
“assume the worst” and your overall score will suffer accordingly.
In general, reviewers will respect honesty and openness, as long as
it is not perceived as an excuse for poor past performance. Further, it
is important that you provide a description of why this past issue is
not an impediment to your present and future excellence. For example:
“During my undergraduate career I was not entirely sure of my dedica-
tion to academics. Because of this indecision I did not perform as well
as could be expected in my classes. However, during my junior year
when I took a course in X, I discovered my passion for Y and as a
result fully applied myself to this newfound love. Now, I am dedicated
to realizing my goals of becoming an independent scientist, as illus-
trated by my significantly improved grades in my final year of under-
graduate and in graduate school.”
Finally, it is very important to remember to write the Personal
Statement keeping in mind the type of training grant for which you
34 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
are applying. For example, a person applying for the F31predoctoral
grant or the F30 MD/PhD grant is at the beginning of their career
training. Therefore, they are at the stage in their development in
which they are obtaining the broad base in knowledge, skills, and sci-
entific thought they need to develop into a strong scientist. In con-
trast, the postdoctoral researcher applying for an F32 fellowship has
already obtained this knowledge. Therefore, their training needs to
focus on the techniques and skills that are essential for them to transi-
tion to an independent academic position, keeping in mind that these
skills are not only technical but also include mentoring, presentation,
laboratory management, and transitioning to independence. Further,
the career goals of the F31 predoctoral applicant (who are training to
become academic scientists) are very different from those of the F30
MD/PhD applicant (who are training to become clinicians/scientists)
and as such they require a different type of training in order to
achieve these goals.
3.1.2 Contributions to Science (Publications)
As with an established investigator on an R-series application, a train-
ing applicant’s scientific productivity is determined by their list of pub-
lications. In the past, an applicant would simply list their publications,
with this list being limited to the top 15 articles most relevant to the
submitted application. However, more recently, this format was chan-
ged to include the section entitled “Contributions to Science ”. The pur-
pose of this change was an attempt to diminish the effect that the
“impact factor ” of a publication may have on the reviewers’ perception
of the importance of the productivity of the applicant. Instead of
merely listing the top most relevant articles, it is now the job of the
applicant to provide a narrative stating exactly how the work that was
performed made a significant contribution to science.
When organizing this section of your Biosketch, each stage of your
training path (i.e., undergraduate, graduate, etc.) should be contained
within a single paragraph. Within this paragraph provide a brief but
descriptive explanation of the work that was performed during this
stage of your training, concluding this description by explicitly stating
the conclusions that were drawn from your work. Wrap up each para-
graph with an explicit statement; “This work is significant because.. .”
and then continue on to state exactly how the conclusions drawn from
your work advanced the field of study, how this work established
35Who Are You?—The Fellowship Applicant
feasibility for subsequent research, or even how this work established a
model system or method essential to the lab in which you worked.
Once you have stated this significance include the statement “The sig-
nificance of this work is indicated through the following publications:”
at which point you provide your bibliography as it pertains the work
you just described in the associated paragraph in a bulleted list, adher-
ing to the NIH standards for references.
It is important to note that the information in the “Contributions to
Science” section of the Biosketch is very similar in nature to the infor-
mation that is included in the “Doctoral Dissertation and Other
Research Experience” section (to be discussed later). However, there is
a distinct and important difference in what the reviewers are evaluating
in each section, which requires you to write each of these with a differ-
ent focus. In the “Doctoral Dissertation and Other Research” section,
you need to focus on several different things: (1) Describe how the
research you performed in the past provided you with a solid research
experience; (2) Illustrate your ability to see a project through to a logi-
cal conclusion; and (3) Provide the basis for the reviewers to evaluate
whether the work you are proposing in the present application is a sig-
nificant departure from your past training experiences. In contrast, in
the “Contributions to Science” section of the Biosketch, you need to
explicitly demonstrate how the work you performed in your previous
training was important enough to make a significant contribution to
science. In other words, you must illustrate to the reviewers that you
are capable of performing what some may consider important
research, research that is capable of changing the way people think
about a field or how people approach a particular question. The differ-
ences in the focus that you provide in the writing of these two sections
may seem like subtle differences, but they are very important differ-
ences. One focus tells the reviewer that what you did before
demonstrates you have the ability to perform the research; the other
tells the reviewer that the work you perform is capable of making a
noticeable contribution to your field of study.
Unlike an established investigator who has been working in their
field for years or even decades, the applicant for a training grant will
not necessarily have an extensive list of peer-reviewed articles. The
reviewers understand this fact and recognize that depending on the
nature of the grant for which you are applying (i.e., F30/F31 vs. F32)
36 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
you may or may not have peer-reviewed publications. For example, a
second-year postdoctoral fellow applying for an F32 training grant will
be expected to have peer-reviewed publications from their graduate
work but not necessarily from their present postdoctoral position.
Along these same lines, a second- or third-year graduate or MD/PhD
student applying for an F31 or F30 will also not necessarily be
expected to have any publications from their present graduate training.
In both cases, though, having peer-reviewed publications, either from
their past work or their present position, will greatly enhance the over-
all perceived quality of the applicant.
Therefore, it is important to consider several alternative forms in
which an applicant can demonstrate their productivity. These alterna-
tive forms can include publications that are in revision or have been
submitted (listing the journal to which they were submitted:
“Manuscript submitted to X”) and manuscripts that are in preparation
(listing the journal to which you intend to submit: “ To be submitted to
X”). It is important to note, however, that these types of publication
listings do not carry nearly the weight as an accepted or published manu-
script. The reason they do not carry as much weight is that unless a
manuscript has been accepted for publication, it has not endured the
rigors of peer review and has not been “ validated” by that process.
Regardless, these manuscripts in development demonstrate to the
reviewers that your work has progressed to a point at which you are
able to prepare it for scrutiny by your peers.
It is also acceptable to include published abstracts, poster presenta-
tions, and invited talks, including all regional, state, national, and/or
international venues in which your work was presented. Although not
published in a journal, which indicates to the reviewers and to the sci-
entific community in general that your work passes the scrutiny of
review, these last three categories demonstrate that you have been pro-
ductive enough to have your work recognized on a larger stage and
accepted for dissemination to the scientific community. Finally, regard-
less of the type of publication, it is highly recommended that you use
bold font for your name and indicate with an asterisk if you are a co-
first author on a publication. By doing this you highlight where your
name falls in the author listing (first author, co-first author, middle
author, etc.), thereby making it easier for the reviewer to determine
your contributions to the work included in that publication.
37Who Are You?—The Fellowship Applicant
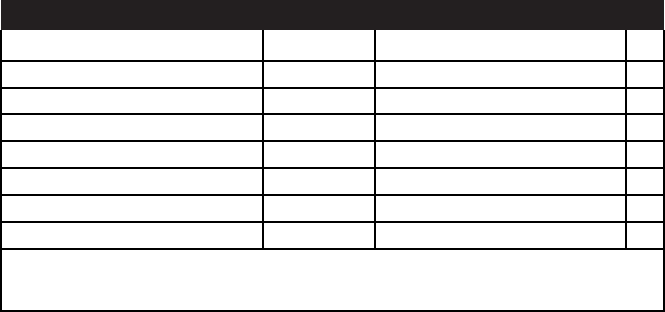
3.1.3 Scholastic Performance
The scholastic performance of the applicant is illustrated by the grades
they achieved throughout their education. The Biosketch form that is
provided by the NIH for the Ruth L. Kirschstein training grant has a
table for entering academic grades and this table and form must be
used. When entering your grades into this table it is important to
remember to include ALL of your grades from your undergraduate
and graduate institutions and not just your science-related grades. The
reviewers want to see the full range of academic capabilities, not sim-
ply in the science-related classes. Also, selecting and choosing which
grades to present may raise a question to the reviewer that there may
not be full disclosure about your academic performance. It is extremely
important to clearly delineate which grades come from which institu-
tion. Remember, the reviewer may be reading your application under
suboptimal conditions (see Chapter 2). Therefore, it is essential to be
as clear as possible to avoid potentially annoying a tired reviewer who
has to work to figure out your academic history. One good rule of
thumb is to place all of the grades from an individual institute into one
column with the name of that institute as a heading (
Table 3.1).
If the list of grades scrolls to a second page retype the name of the
institute within the appropriate column on the new page. If you have
taken a course that was not graded on an ABC scale, identify how
the course was graded (Pass/Fail, Honors/High Pass/Pass, etc.) and
what criterion was used to derive the different grade rankings. Finally,
Table 3.1 The Listing of Academic Grades
The University of Delaware Johns Hopkins University
General Chemistry A Graduate Biochemistry B
Organic Chemistry B Biophysics B
Physical Chemistry C Molecular Biology A
Instrumental Analysis B Bioorganic Mechanisms B
German A Immunology A
Seminar P
Anatomy and Physiology HP
Some courses at Johns Hopkins University are graded on a Pass/Fail basis (P/F). Medical School classes are
graded as Honors (H), High Pass (HP), and Pass (P), which correlate to scores of 90%100%, 80%90%,
and 70%80%, respectively.
38 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
include your GRE or MCAT scores and if you are an MD/PhD stu-
dent it may even be helpful to include your Step 1 Certification results.
Remember, the reviewers are looking for the cream of the crop. It
will be the applicants with 4.0 GPAs, GRE scores or MCAT scores in
the top percentiles who are ranked highly in terms of their scholastic
performance. If you have an academic history in which you’ve earned
a solid mix of As and Bs you will also be considered an exceptional
applicant. One C will raise eyebrows and potentially affect your score
while more than one C (or even lower) will significantly affect your
score. It is important to remember that this is your history. It is fin-
ished and there is nothing you can do to change it. However, you must
address this issue directly, which can be done in several different ways.
First, the focus of these training grants is to assist in the development
of an independent researcher, where capabilities and excellence in the
lab many times carry more weight than past academic grades.
Therefore, have one or more of your references explicitly state that
your academic record does not truly reflect your capabilities in the
laboratory. Second, many times an applicant had a poor academic
performance due to difficult personal issues or simply because they did
not know what they wanted to do with their career. If this is the case,
concisely and tactfully describe this issue in the Personal Statement of
the Biosketch (see above). The reviewers do not want to know the
nitty-gritty details of your personal life. However, if your personal life
(e.g., taking care of an ailing family member, working two jobs to pay
for school, unexpected health issues) impacted your ability to perform
to your optimal capacity, it is essential that the reviewers are aware of
this fact. Be certain, too, to point out that those issues are no longer a
factor in your life and you have focused on your career goals. Finally,
many times an applicant has poor grades in undergraduate but then
improves significantly in their graduate work. If this is the case, make
an explicit point of this in your Personal Statement. As with the second
point, this indicates to the reviewers that you now have your act
together and are able to focus your attentions on your present
training.
3.2 APPLICANT’S BACKGROU ND (6 PAGES MAXIMUM)
Recently the NIH created a new section for the Ruth L. Kirschstein
training grants called “Applicant’s Background”. Although this is a
39Who Are You?—The Fellowship Applicant
newly created section of the application, the information contained
within it is not new and in fact groups together three formerly indepen-
dent sections of the application package: (1) Doctoral Dissertation and
Other Research Experience (formerly entitled Previous Research
Experience); (2) Goals for Fellowship Training and Career; and (3)
Activities Planned Under This Award. Further, instead of giving an
explicit page limitation for each individual component, which when
combined was four pages maximum, it expanded this page limit to a
six-page maximum for the entire new section. Regardless, the informa-
tion contained within this section of the application still serves the
same purpose; to provide the reviewer with information about your
past experiences, how these experiences contributed to your overall
goals for your training and future, and how your proposed training
will inform the activities you plan to undertake during your training.
3.2.1 Doctoral Dissertation and Other Research
In addition to a solid academic record and good productivity, the
reviewers want to see that you have previous research experience. This
previous experience covers all of your previous work, including high
school science internships, summer research programs, undergraduate
research projects, and graduate school rotation projects. It is also nec-
essary to describe your doctoral dissertation research up to your pres-
ent point of education (if you are a predoctoral or MD/PhD student)
or your postdoctoral research to the point at which you are writing the
application. This description of research experience serves several pur-
poses in the evaluation of the applicant. First, it shows how much
research experience you had before entering your present training posi-
tion. This information is important because the extent of your previous
research experience will indicate how much training you will require in
your present position and will also provide a measure for the reviewers
to use when evaluating your productivity through publications. Again,
remember the type of training plan for which you are writing. While
definitely a plus to have experience, extensive research exposure is not
as essential for predoctoral and MD/PhD students as it is with post-
doctoral researchers, who will have completed a thesis project. Part of
the assumption with the predoctoral candidates is that they are in the
training program to learn how to perform academic research.
In addition to detailing the extent of your previous research experi-
ence, this section will also provide information on the scope and
40 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
variety of your previous experience. The reviewers use this information
to determine not only the breadth of your experience but also how
much your present training will be a departure from your previous
work. There are instances where the applicant has a fairly extensive
research history. However, all of their research is in the same field.
For example, an applicant performed undergraduate research examin-
ing neurodegeneration using cell culture techniques. They then pro-
gressed to their predoctoral work where they did a similar type of
research in an identical model system but examining a slightly different
molecular mechanism. They then apply for a postdoctoral training
grant in which their project is also examining neurodegeneration in a
cellular system. While there is nothing wrong with having a very
focused knowledge of exactly what type of research interests you, the
continuation of a homogenous focus of research throughout your
entire training history (i.e., neurodegeneration in a cellular system) will
not be perceived as providing any additional training opportunities. In
this scenario, it is imperative that you point out in your Personal
Statement and Goals sections why you chose to pursue such a focused
research interest, which can be perceived as limiting your training
potential, and explicitly state how the present training will truly
broaden your experience and provide you with novel training opportu-
nities. Further, have your sponsor explicitly state in their training plan
(discussed in Chapter 4) how the present program will provide you
new training.
The previous scenario is not intended to imply that having a
defined research interest is a negative thing. However, it is important
to remember that in addition to focusing on the training you will
receive, this application also examines the maturity of the applicant.
Therefore, having explicit ambitions to work in a specific field requires
clearly defined reasons describing why you have this apparent “hyper-
focus.” Many times reviewers see it as a positive when an applicant
does, in fact, have a very clear picture of their research interests and
goals and their research experience supports this focused ambition.
However, in general what the reviewers are looking for is that you
will, in fact, gain new training experiences with the money provided
by the training grant. These new experiences can be obtained by
working in the same field but in a completely different model system.
For example, an applicant is interested in the role of signaling
41Who Are You?—The Fellowship Applicant
pathways in the development of cancer. They pursued this interest in
their predoctoral work using a cellular model system. They have now
progressed on to their postdoctoral work where they are working in
the same field, but have now moved to an animal model system.
Although the research focus is the same, they will obtain invaluable
experience working with animals and learning how to relate results
found in the animals to what is known in cells. Another example
would be a case where an applicant performed extensive undergradu-
ate research examining the effects of alcohol on regulating gene
expression in muscle development using a cellular model. Their pres-
ent dissertation research is now working on the same question in an
identical model system. However, instead of examining the molecular
mechanisms at work (e.g., transcriptional regulation) they are now
using genomics and bioinformatics to look at global gene expression
differences and effects. Again, the use of significantly different techni-
ques and cutting edge technology will provide invaluable training in
different forms of analysis.
When writing about your previous research experience, it is essen-
tial to break down your descriptions into distinct, identifiable sections
or paragraphs based on the time period in which you did the work
(high school internship, undergraduate honors project, dissertation
work, etc.) and the mentor for whom you worked at the time. It is best
to present these sections in chronological order from earliest to the
most recent. Within each section you need to explicitly describe the
research focus of the lab where you worked and what you did during
this experience: “The research of Dr. X involves... During my time in
this laboratory my project focused on determining...” Tell them how
you went about addressing the question of your research and then
wrap up the section by explicitly stating the conclusions that you were
able to draw from your work; “Through this work I was able to dem-
onstrate that X causes Y, which allowed us to conclude Z.” Also, it is
extremely important to state whether this work resulted in a peer-
reviewed publication, published abstract, or poster presentation in
which you were included as an author. “This work was of a signifi-
cance that allowed my inclusion as an author on...” After you make
this statement provide them with a reference, even if that reference is
included in your Biosketch. Remember, the reviewer may not want to
have to return to your Biosketch to find the reference to which you are
referring.
42 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
As stated above, the information included within this section is very
similar to the information provided in the “Contributions to Science”
section of the Biosketch. However, whereas the focus of the writing in
the Biosketch needs to inform the reviewer of the importance and sig-
nificance of your previous research, the focus of the writing in this sec-
tion needs to elucidate the amount and nature of your previous
experience. In addition to telling the reviewers how much research
experience you have and the variety of research training to which you
were exposed, this section also demonstrates that you have a history of
being able to tackle a project and move it to a logical conclusion at a
level that is accepted through peer review or public dissemination.
3.2.2 Goals for Fellowship Training and Career
For an applicant to be considered truly exceptional, not only must
they have a solid academic record and history of productivity, but they
must also possess a mature and concrete view of their future career
plans and they must be able to clearly communicate how the present
training will help them achieve these goals. The section “Goals for
Fellowship Training and Career” is the section in which the applicant
does just that. When stating your career goals it is important to be spe-
cific and to clearly state what your career goals are. For example,
some applicants will state “My career goal is to run an independent
lab in a high-power institute.” This statement is vague and tells the
reviewer nothing about what field of research inspires and interests
you. A better statement would be: “My ultimate career goal is to
become an independent researcher at an academic institution and to
establish a laboratory that studies molecular pathways that contribute
to human disease.” While still being somewhat general, it provides a
more mature explanation of where you see yourself (an academic insti-
tution), what type of research you would like to perform (examining
molecular pathways), and what aspect of overall health-related work
(human diseases). If possible, it is better to further define the molecular
or biological process that interests you and how that process contri-
butes to a more specific health field (e.g., understanding the role of sig-
nal transduction pathways in neurodegenerative disease).
Once you have stated your career goals, provide a clear explanation
of how the present training and sponsor will help you achieve these
goals: “By providing me with extensive training in technical and inves-
tigatory aspects related to molecular pathways involved in
43Who Are You?—The Fellowship Applicant
neurodegenerative disease, this project and sponsor fits perfectly into
my future goals of using my research skills to...” Follow this state-
ment by describing the field in which the sponsor works and how that
fits your goals, and any explicit technical training that you will receive
(e.g., Western blot analysis, DNA isolation, analysis of signal trans-
duction pathways, etc.). It is very important to remember that training
does not solely involve the technical aspects of science. Many applica-
tions are criticized for not describing the training they will receive in
the nontechnical aspects of academic research, which include experi-
mental design, results analysis, manuscript/grant preparation, presenta-
tion skills, and networking. These are skills that are essential for a
trainee to learn in order to be truly successful as an independent scien-
tist and are usually learned directly from the sponsor. As such, these
aspects of training must be explicitly stated. Further, it is important
for postdoctoral fellows to describe how the present training and spon-
sor will help them transition to an independent career and whether
they will be allowed to develop a project to take with them to initiate
their independent research. It should be noted that the sponsor must
also include a similar statement in the Training Plan section of the
Sponsor/Co-Sponsor section (see Chapter 4) that confirms that they
will, in fact, allow the postdoctoral trainee to take with them to begin
their independent career. Along these same lines, applicants for the
MD/PhD must detail how the present training will introduce them to
the clinical aspects of training that will enable them to become an
exceptional clinician-scientist.
Another aspect that must be discussed in this section is the environ-
ment in which the training will take place and how the environment will
contribute to you achieving your career goals. The environment will
provide access to various different resources that will greatly enhance
and enrich a training program, including access to cutting-edge techno-
logies, proximity to other institutes to foster collaborations and net-
working, and journal clubs and/or seminar series that will expose the
applicant to invited speakers. Further, if you are a student, it is essential
to describe how the Departmental program will provide essential
training to obtain your career goals: “The training program set forth by
the Department of Genetics will contribute to my long-term goals by
providing me with a strong and diverse base of knowledge through a
curriculum that includes...” Again, if you are an MD/PhD applicant,
you must detail how the MD/PhD program at your institute is perfect
44 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
for your training goals and how the resources at your institution will
enable exposure to clinical-research experiences. Finally, if you are a
postdoctoral researcher, it is beneficial to include a description of any
career development programs that may be present or what measures
your department and/or mentor will take to assist you in a transition to
independence. As stated above, it is essential that similar comments
appear in the Training Plan section of the Sponsor/Co-Sponsor infor-
mation attesting to their assistance in helping the postdoctoral trainee
transition to independence. Remember, regardless of which training
grant you are submitting, you are selling yourself and everything about
the training program, which includes the sponsor, the department, the
educational program, and the institutional environment. You want to
convince the reviewers that as a whole this program is perfect for you to
receive an exceptional training. They want to see a multifaceted pro-
gram that will give you all of the aspects of training that are required to
help you achieve a clearly defined long-term career goal.
As with the Personal Statement, it is important to remember the
type of training grant for which you are applying. With the postdoc-
toral F32, the reviewers will want to see a more discretely defined goal
than in the predoctoral and/or MD/PhD training grants, because the
postdoctoral years are where an investigator truly hones their skills in
their field of interest. Also, the postdoctoral researcher is learning the
skills needed in order to transition to an independent faculty position.
Therefore, it is essential that a description of how the present training
will help them achieve that goal of independence (including laboratory
management, mentoring, and having a project for them to take with
them to establish their independent laboratory) must be included. For
example: “Dr. X is dedicated to preparing me for an independent posi-
tion by teaching me different aspects of laboratory management.
Further, through several discussions, Dr. X has agreed to allow me to
develop part of my work into a project to take with me to establish an
independent lab. ” This sentiment must then be mirrored and included
in the sponsor’s Training Plan.
In contrast, reviewers understand that predoctoral students are
learning how to think like a scientist and most likely the focus of their
doctoral dissertation work will not necessarily be the field in which
they work in their independent careers. Therefore, the description of
the goals for the present training for predoctoral students should focus
45Who Are You?—The Fellowship Applicant
more on the training they will receive to think like a scientist and how
to develop, analyze, and present their work and be less on the exact
field of interest. Finally, when applying for an F30 MD/PhD grant the
reviewers usually expect to see a clinical slant in the writing consistent
with the potential for clinical and/or translational research in the
future. Therefore, the description of the goals for these grant applica-
tions must include descriptions on how the training they will receive
has clinical aspects involved and how these clinical threads contribute
to their overall goals of becoming a clinician-scientist.
3.2.3 Activities Planned Under This Award
The reviewers want to see that you have a distinct and realistic per-
spective on the time frame in which your training will progress and
that the proposed training will be able to be accomplished within the
funding period. In the section “Activities Planned Under This Award”
you break down each year of your training into a “percent effort” indi-
cating what percent of your time will be dedicated to different aspects
of your training. These different aspects of training include research,
professional development, teaching/mentoring, and clinical (if appro-
priate). Present the breakdown of the percent efforts in a table with
columns for each of the applicable pursuits and a row for each year of
your training (
Table 3.2). It is important that you critically evaluate
the time you will spend in each of these pursuits, remembering that the
primary role of your training is research. Further, the percent efforts
should change as the training period progresses. For example, a post-
doctoral researcher may have 5% effort in professional development in
the first 2 years. However, the final year of training will also involve
the search for an independent faculty position, which involves the
preparation of your application and going on job interviews.
Therefore, your professional development will increase slightly in the
final year.
Table 3.2 The Delineation of Activities Planned Under the Award for a Postdoctoral
Trainee
Research (%) Professional Development (%) Teaching/Mentoring (%) Clinical (%)
Year 1 90 5 5 0
Year 2 90 5 5 0
Year 3 85 10 5 0
46 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
The table is followed by a description of what activities you con-
sider to be part of the overall headings. Begin this section with a state-
ment reminding the reviewers that you understand how the training to
become an independent investigator involves more than simply the
technical aspects of research: “My development into an independent
investigator involves undertaking activities not only in the technical
aspects of my chosen field but also in the realm of professional devel-
opment, teaching, and transitioning to independence. Therefore, I will
undertake the following activities to achieve my training.” While it is
not necessary to break down the research into excruciating detail, it is
helpful to indicate how long you predict each independent Specific
Aim to take to complete. Keep in mind, the reviewers are all investiga-
tors and they understand that this is science and a time prediction is
just that... a prediction. “I will focus on Experiments 13 of Specific
Aim 1 in the first 6 months of my training. During this time I will also
begin the breeding of mice to generate the animal model system
described in Specific Aim 2.”
When discussing the professional development aspects of your train-
ing, be sure to explicitly and clearly define what you consider to be
professional development. Professional development should encompass
the writing of manuscripts and grants, presentation of your work, net-
working, and attendance at seminars and classes important to your
education. For postdoctoral fellows, it is essential that the professional
development include the learning of laboratory management and the
search for an independent faculty position. Finally, the reviewers are
expecting that you will progress on to an academic career. Therefore,
most study section members like to see that you will have experience
in the mentoring of students and possibly limited teaching of classes.
3.3 SELECTION OF SPONSOR AND INSTITUTE
(1 PAGE MAXIMUM)
The sponsor, or the mentor/advisor, is the person who has the greatest
influence on the direct training of an applicant. As such, the reviewers
want to see that the applicant has given careful thought into how they
chose this person with whom to conduct their training. Further, the
department and institute will also contribute to the overall training of
an individual. In the “Selection of Sponsor and Institute” section, you
will explicitly and clearly describe why you selected the sponsor,
47Who Are You?—The Fellowship Applicant
department, and institute in which to perform your training. You need
to state exactly what it was about each of these three components that
made you feel that this would be the “perfect convergence” of factors
to give the best training for you as an individual. In some ways, this
section contains similar information as the “Goals for Fellowship
Training and Career” section. In both sections you describe how your
training will help you achieve your goals. However, while the focus of
the Goals section is to talk about how the overall training, which
includes the sponsor, department, and institute, will help you achieve
your goals, the “Selection of Sponsor and Institute” section focuses on
the unique qualities that your advisor, the department, and the institute
have that will give you the optimal training you need.
When writing this section, as with all of the parts of the application,
you want to be direct and to the point to make it as easy as possible
for the reviewer to see the exceptional nature of your choices and that
your selections were mature and well informed. In this section it is also
useful to break each of the topics (i.e., institute, department, and spon-
sor) into their individual paragraphs with each paragraph beginning
with a variation of the following “catch phrases.”“I chose to pursue
my PhD (or MD/PhD or postdoctoral research) at institute X
because...” Follow this introductory sentence with explicit reasons for
why this institute fits your needs: They have an exceptional educational
program that provides a solid basis from which to build your training;
they provide diverse research opportunities for a student; they have an
interdisciplinary program to enhance your research experiences; a
strong collaborative environment exists to promote collaborations, etc.
Along these same lines, be very explicit about why you chose a particu-
lar department; “I chose to undergo my training in Department Y
because...” Again follow this statement with distinct and clear reasons
(e.g., the type of research going on in the department, the quality of
the research, the supportive caring faculty, the departmental educa-
tional program, etc.).
Most importantly, describe your reasons for working with the men-
tor; “I chose to work with Dr. Z for multiple reasons. Among these
are...” When describing your reasons, in particular with your sponsor,
remember that the training involves more than just the technical
aspects of science. Also it is important to remember that your mentor
will be the person who is most influential in your training and will be
48 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
the one most responsible for molding who you become as a scientist.
For many people the mentormentee relationship is a personal one,
and therefore, in addition to describing how the sponsor’s research fits
your scientific goals, you must describe how their particular mentoring
style is suited for you.
When writing the paragraph about the selection of the sponsor, it
may be helpful to think of some of the following questions, determine
if these questions apply to you, and if they do, how you would answer
them. (1) Does your sponsor have the ability to be more hands-on,
which means there will be more extensive interactions between the two
of you? This may be important for the predoctoral and/or MD/PhD
student who are beginning their training. (2) Does your sponsor take a
more distant approach, which means you will be left more to your
own devices? This style may be more important for the postdoctoral
researcher where independence is required. (3) Does your sponsor have
a unique teaching style that has proven results? This is evidenced
through the level and quality of career placement that the previous
trainees obtained after leaving the laboratory. (4) How does the spon-
sor teach? Do they use more of a “Socratic” method, which leads you
to the question versus a more didactic method, which tells you the
answer directly? In addition to thinking about the sponsor and how
their mentoring style meshes with how you learn, it is also important
to talk about the laboratory environment. Is it a large lab, giving you
many opportunities to interact and learn from others? Is it a small lab
that creates a closer, more nurturing environment? Finally, after you
have discussed all of these characteristics it is essential that you swing
it back to focus on you, the trainee, and describe exactly why all of
these characteristics that are unique to your sponsor and laboratory
environment are perfect for you as an individual and your specific
training needs.
Finally, some training grants require that the applicant has a
cosponsor or collaborator to supplement the perceived weaknesses of a
junior faculty or to provide scientific expertise for a particular aspect
of the project (to be discussed in Chapter 4). If you include a cospon-
sor or collaborator, it is necessary for you to describe why you chose
the specific person you did for this role. As with the sponsor descrip-
tion, explicitly state what qualities or expertise the cosponsor will
bring. “Because my sponsor is a junior faculty and has limited training
49Who Are You?—The Fellowship Applicant
experience, I have chosen Dr. X as a cosponsor. Dr. X has a long his-
tory of training students and therefore will be able to...” However, it
is not sufficient to simply pick a cosponsor because they simply “fill a
perceived gap” in your sponsor’s qualifications. It is important that
they also have technical or scientific expertise to complement your
project. “In addition, Dr. X has worked in the field of Z for 17 years,
as evidenced by his publication record, and will...” After this state-
ment, describe how the cosponsor will be instrumental in providing
you the training you need as it relates to your project and your career
goals. It is also beneficial to mention that the cosponsor will serve on
your thesis committee (if you are a predoctoral or MD/PhD student)
or on your advisory committee (if you are a postdoctoral fellow).
Further, describe how the cosponsor will assist you in experimental
design, results analysis, manuscript preparation, etc. What is essential
when describing the cosponsor is that their contributions to your train-
ing must seamlessly fit into the overall training plan. The reviewers
want to see that the cosponsor or collaborator will be integral to your
training and not simply a tangential figure placed there to “appease”
previous critiques or to “pad” your grant application.
3.4 LETTERS OF RECOMMENDATION (3 REFERENCES
REQUIRED)
Your letters of recommendation will provide the reviewers with an
independent evaluation of your capabilities. These letters also provide
a description of a history of excellence, suggesting to the reviewers that
this history will translate into a solid potential for your future success
as an independent investigator. Therefore, it is essential that you
choose the people who will serve as your references carefully. One of
the biggest mistakes applicants make is selecting references that all
derive from the same institute, if not even the same department, as
where the present training is taking place. The department and insti-
tute where you are presently working have a vested interest in your
success and as such would be expected to write solid letters of support.
Therefore, a useful guideline when selecting references is as follows: if
you have performed research at another institute (i.e., graduate work,
summer internships, undergraduate research, etc.), select your previous
mentor to serve as at least one of your references. If you have per-
formed research at several different institutes, then request letters of
50 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
support from several of these mentors. It is also advisable to select the
third reference from within your institute but from a faculty member
that is outside of your present department. If possible, make sure that
this person serves on your thesis or advisory committee so they are
capable of commenting directly on your potential as an independent
researcher. It is possible that an applicant, particularly a predoctoral
student, may not have performed research as an undergraduate. If this
is the case, request a letter of support from a faculty member at the
undergraduate institute who is capable of commenting on your poten-
tial as an independent researcher. Finally, if you are an MD/PhD stu-
dent it would be beneficial to get a letter of support from a clinician or
someone who can comment on your potential as an independent
clinician-scientist, the easiest idea for this being the director of the
MD/PhD program. What these guidelines illustrate is the importance
of examining your educational and research history and carefully
selecting references from each stage of your development to highlight a
track record of excellence and a diverse consensus on your abilities as
an independent researcher.
When you are deciding on whom to select to serve as references, be
sure to select people that you know will write you solid, strong letters
of recommendation. This scenario is similar to the adage a defense
lawyer takes; never ask a witness a question for which you don’t
already know the answer. A weak or poorly written letter from a refer-
ence will significantly affect how well an applicant will be reviewed.
Further, when you contact your references be sure to explicitly ask
them to comment on your potential for a successful career as an inde-
pendent researcher (F31 and F32 applications) or physician scientist
(F30 application). If as discussed above you have a poor academic
history and this history is not necessarily an indication of your capabil-
ities as a scientist or resulted from external personal issues that were
out of your control, select a reference that can explicitly comment on
this fact and specifically ask them to discuss this in their letter. For
example, an applicant may have had poor grades during several seme-
sters of their undergraduate work. However, their passion was at the
bench, where their true capabilities came through, a fact on which the
undergraduate mentor can elaborate. Or, if these poor grades resulted
from personal tragedy, illness, or unusual circumstances, it is impor-
tant that the mentor from the undergraduate work makes these facts
abundantly clear and explicitly state that the classroom grades in no
51Who Are You?—The Fellowship Applicant
way represent the true capability of the applicant. Reviewers usually
place more weight on the laboratory and scientific skills of the
applicant, particularly when references from all aspects of an appli-
cant’s training career independently concur on this fact.
The applicant does not submit the letters of reference and more
importantly, the applicant is not allowed to see these le tters. Instead
the applicant must arrange to have the referees submit their r ecom-
mendations thr ough eRA Commons at the f ollowing web address:
(
https://public.era.nih.gov/commons/public/reference/
submitReferenceLetter .do?mode 5 new
). Because the references are
being submitted through the eRA Commons and not Grants.gov, the
applicant must provide the referees with specific information including
their eRA Commons user ID, their last name, and the Funding
Opportunity Announcement number. Previously, in addition to the let-
ter of recommendation the referee was required to rate the applicant
on a scale of 15 for their excellence in a list of 12 individual catego-
ries. However, this is no longer the case. Now the referees simply
upload a pdf file containing their signed letter of recommendation on
letterhead. It is important to note that these references are due by the
application deadline and if not received by that time may result in the
application being administratively returned without review.
3.5 RESPECTIVE CONTRIBUTIONS (1 PAGE MAXIMUM)
All reviewers know, and expect, that you did not construct this appli-
cation in a void independent of your sponsor. Further, they realize
that your project did not simply materialize independent of the work
ongoing in the laboratory in which you are working. Therefore, this
section serves as the place where you tell the reviewers exactly what
contributions each individual person made to the development of this
application and will make in the future work associated with this
grant. Some key phrases that may help in the writing of this section
are as follows:
•“The development of the research plan put forth in this proposal
was developed as a collaboration between Dr. X and myself.”
•“The specific aims that will be undertaken derived from small facets
of ongoing studies within the lab.”
52 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
•“This plan was developed from extensive literature research and pre-
liminary data performed by myself.”
•“Frequent one-on-one meetings with Dr. X helped me develop this
plan.”
•“I was responsible for writing the initial draft of this proposal, which
then underwent multiple revisions, with the assistance of Dr. X.”
•“I will be the primary investigator in accomplishing the work
described in this proposal. I will carry out the development of
experiments and analysis of results with guidance from Dr. X.”
Although this section is probably one of the least scrutinized when
evaluating the applicant, it is extremely important that the same care
and meticulousness that was used to write all of the other sections be
used to write this section, too.
3.6 THE SECOND TIME AROUND — PERFORMING A SECOND
POSTDOCTORAL TRAINING
On occasion an applicant who is performing their second postdoctoral
training will submit a Ruth L. Kirschstein grant. While this is not nec-
essarily a bad thing in terms of a person’s career development, this par-
ticular type of applicant will have to provide detailed information
describing their reasons for undertaking a second postdoctoral posi-
tion. It is misguided for an applicant to think that the reviewers will
not realize that this is their second postdoctoral experience. The basic
fact is that reviewers scrutinize applications for fine details and will in
fact notice that an applicant is in their second postdoctoral position
and will want to know why a second postdoctoral training period was
required. If there is no explicit description, the reviewers may think
there is something being hidden from them, which will adversely affect
the overall score. There are a multitude of reasons why a person would
elect to perform a second postdoctoral training: The applicant’s
research interests developed in a direction that fell outside the realm of
expertise in their present laboratory and they needed additional train-
ing; personal issues developed between them and their advisor creating
an adverse training environment, etc. However, regardless of the rea-
son, these issues must be addressed either directly or tactfully.
The issue of the second postdoctoral position will need to be
addressed in almost every component that relates to describing the
53Who Are You?—The Fellowship Applicant
applicant. In the Biosketch Personal Statement, be forthcoming with
the fact that this is your second postdoctoral position. For example:
“During my first postdoctoral training period my research unexpect-
edly introduced me to field X, which I found to be extremely
interesting. Therefore, I decided to perform a second postdoctoral
training in this new field to gain more in depth exposure and hands-on
experience.” Along these lines, this same point must be discussed and
expanded in the Goals for Training and Career section, as this is a dis-
tinct, and unexpected, change in your career path. Further, this change
will also require that you justify the selection of your new sponsor with
explicit discussion in the Selection of Sponsor component and a discus-
sion of the research you undertook in your first postdoctoral position
in the Previous Research Experience component. Finally, if you left
your first postdoctoral position on good terms it is essential for you to
have a letter of recommendation from your first postdoctoral advisor,
in which that person reiterates why it was essential for you to obtain
further postdoctoral training in another lab. However, if the second
scenario discussed above, in which you did not leave the first postdoc-
toral training lab on good terms, is the case, do not include a letter of
recommendation from that advisor (as they can not necessarily be
counted on to provide you with a solid recommendation) but provide
a tactful explanation for this in the Personal Statement of the
Biosketch for why their recommendation is not being included. In this
latter scenario it is beneficial to also have your present mentor
discuss this in their recommendation of you, which is included in
the Sponsor’s Information section (discussed in Chapter 4).
54 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant

CHAPTER
4
4
Who’s the Boss?—Sponsor, Collaborators, and
Consultants
The sponsor, or the mentor, is probably the most influential person
who will be involved in the training of the applicant. Therefore, a
detailed description of the sponsor’s qualifications as a scientist and as
a mentor, along with an explicit outline of the individual training plan
they have developed for the applicant, is extremely important for the
evaluation of the training potential of an application. Reviewers use
several different components to evaluate the overall excellence of a
sponsor, including their expertise in the field of study of the applicant’s
research project, their productivity, their funding history, the number
of previous trainees they have produced and the quality of their train-
ing, the number of trainees to be supervised during the training period
of the applicant, and the training plan for the applicant. As with all
aspects of the Ruth L. Kirschstein training grants, it is important to
understand the purpose of each of these components in order to con-
vince the reviewers that the chosen sponsor will provide you with the
best individualized training possible in order for you to achieve your
career goals.
4.1 BIOSKETCH (5 PAGES MAXIMUM)
The sponsor’s expertise and productivity in the field of study of the
applicant’s research project are illustrated through the Biosketch. In
general, the sponsor’s Biosketch is identical to those used for the R-
series research grants (R01, R21, and R03). Just as for the R-series
grants, the sponsor needs to provide enough information in the
Personal Statement to give the reviewers assurance that they are truly
qualified to direct the scientific portion of the application. In addition,
it is extremely important for the sponsor to provide information that
discusses their dedication to mentoring and teaching and their success
in both areas. The dedication of the sponsor to teaching and/or men-
toring can be described with examples of their teaching duties, serving
A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant. DOI: https://doi.org/10.1016/B978-0-12-815336-9.00004-1
© 2018 Elsevier Inc. All rights reserved.
on departmental or institutional education committees, etc. The quality
or excellence of their mentoring skills, in turn, can be illustrated by
using explicit statements that describe where their previous trainees are
presently working. For example: “The quality of my mentoring is illus-
trated through the fact that several of my previous students progressed
on to perform their postdoctoral work at top rated institutions, such as
Harvard and Stanford Universities.” Finally, in addition to supporting
the qualification of the sponsor’s expertise, their productivity is evident
through the number of publications, the topics of these publications,
and the quality of these publications, just as described for the applicant
(see Chapter 3).
4.2 SPONSOR AND COSPONS OR INFORMATION (6 PAGES
MAXIMUM)
Probably the most important part of the application for the sponsor is
the section: “Sponsor and Cosponsor Information.” In this section, the
sponsor provides detailed information about their history and training
qualifications, the training environment, and the detailed training plan
that they have developed for the applicant. This section is broken
down into five parts: Research Support Available; Sponsor’s Previous
Trainees; Training Plan, Environment, Research Facilities; Number of
Fellows/Trainees to be Supervised during the Fellowship; and
Applicant’s Qualifications and Potential for a Research Career.
4.2.1 Research Support Available
One of the most common questions asked by faculty and trainees who
are inexperienced with the submission of these training grants is this: If
you are applying for funding to support your training, why does the
sponsor have to demonstrate that they have funding in place to sup-
port the trainee? What many people do not realize is that the Ruth L.
Kirschstein grants will support the trainee’s tuition and fees, stipend,
and a few thousand dollars a year to cover incidentals (travel, supplies,
etc.). Therefore, there are insufficient funds within the award to sup-
port the day-to-day research activities of the trainee and their science.
The listing of the sponsor’s available research support demonstrates
that they have money in place, for the duration of the training period,
to provide the supplies and reagents needed for the successful comple-
tion of the research plan.
56 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
It is important that you provide information on all present and
pending research support. For each of the grants for which you are
funded indicate the grant source; the ID number (which tells the
reviewers what type of grant you have); the title of the grant; your role
on the grant (PI, co-PI, etc.); the dates that the grant is active; and the
annual direct costs. This information needs to be presented in a clear,
concise, and direct manner, which is most easily done by presenting it
in a tabular format (
Table 4.1).
If a grant will end during the training period and you have submit-
ted a competitive renewal, this must be stated. If you have a pending
grant that has been reviewed and received a solid score, provide this
information, too. If you are a junior faculty member and have yet to
obtain significant external funding, but are supported through institu-
tional start-up money, this information must be given accompanied by
a letter of support from the Department Chair confirming that funds
are guaranteed and that these funds are available to support the
trainee’s research. In general, the reviewers want to see that you have
financial support available, or that you are in a solid position to
continue funding, for the duration of the applicant’s training period.
4.2.2 Sponsor’s Previous Fellows/Trainees
Every single person who sits on the study section are themselves estab-
lished investigators at academic institutions. As a prerequisite to sit on
an National Institutes of Health (NIH) study section, these people
must have successfully obtained funding from the NIH. Therefore,
they are sufficiently senior in their career to have trained a number of
students and/or postdoctoral fellows. Because of their own experience,
they realize that your ability to train students is an “on the job”
learning process and will mature and become fine-tuned with the more
trainees you mentor. Granted, some people have the gift of being able
to teach and mentor. However, even these gifted people have a level of
Table 4.1 The Presentation of Research Support
Grant
Source
ID Number Title PI Dates Annual
Direct Costs
NIH/NCI R01CA123456 Mechanism of regulation
for the oncogenic
transcription factor in
melanoma
Smith
(PI)
6/01/183/31/23 $207,500
57Who’s the Boss?—Sponsor, Collaborators, and Consultants
naiveté when they begin their independent career that naturally
prevents them from being able to provide an optimal mentoring envi-
ronment. Therefore, the reviewers want to see that you have a history
of successfully training people to productive careers at solid academic
institutions.
In the “Sponsor’s Previous Fellows/Trainees” section, explicitly
state the number of previous predoctoral trainees and the number of
previous postdoctoral trainees. Do not hide this information in a para-
graph that describes your own personal training history. Instead make
this information prominent and easy for the reviewer to see:
• Number of previous predoctoral trainees—7
• Number of previous postdoctoral trainees—4
In addition, this section requires that you provide a list of five of
your representative previous trainees and describe where they are
presently in their career. Telling the reviewer where your trainees pro-
gressed in their careers indicates the quality of the training each person
received under your guidance. As with the research support, this infor-
mation is best presented in tabular format where you include the name
of the trainee, the years they trained in your lab, their position in the
lab, and their current position (including position title and institute)
(
Table 4.2).
If you have more than five previous trainees, carefully select which
of these trainees you list in order to highlight the quality of your train-
ing. Further, when selecting which trainees you list, pay attention to
Table 4.2 The Presentation of Previous Trainees
Name Years in
Training
Position in
Lab
Current Position
Alvin Siddy, MD, PhD 200409 MD/PhD
student
Senior resident in Pediatric Medical Genetics,
Detroit Medical Center, Children’s Hospital of
Michigan, Detroit, MI
Kathy Dent, PhD 200510 Graduate
student
Postdoctoral researcher, Center for Human
Genetics Research, Harvard University, Boston,
MA
Mamie Abrigail, PhD 200711 Graduate
student
Assistant Professor, Department of Zoology, Yale
University, New Haven, CT
Kevin Johnson, PhD 200509 Postdoctoral
researcher
Assistant Professor, Department of Chemistry,
Loyola University, Chicago, IL
58 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
the type of grant for which your trainee is applying. If they are apply-
ing for an F32 postdoctoral fellowship, you will want to list primarily
your postdoctoral trainees. If they are applying for an F31 or F30 pre-
doctoral fellowship you will want to primarily list your predoctoral
trainees. The reviewers understand that different types of mentoring
are required for the different stages of training. Therefore, just because
a sponsor has a history of excellence in training predoctoral students,
it does not necessarily follow that this person will also be a good men-
tor to postdoctoral fellows and vice versa. Finally, try to select previ-
ous trainees that have stayed within the United States and are
presently at academic institutions. While it is not necessarily a major
issue that trainees are presently working in foreign countries, the basic
line of thought is that these are United States federal tax dollars that
are being used to support the training. Whenever a trainee leaves the
country it may be implicitly felt, and sometimes even explicitly stated
in study section, that federal tax dollars went to support a foreign
economy and intellectual environment.
4.2.3 Training Plan
In the Training Plan the sponsor supplies a detailed description of the
exact methods to be used to train the applicant. This plan must be indi-
vidualized and tailor-made for each applicant. As stated above, the
reviewers have trained their fair share of individuals and realize that
every person needs a different style and method of training in order for
them to achieve their maximal capabilities. Also, postdoctoral fellows
require a different style of training than predoctoral students. Along
these same lines MD/PhD predoctoral students need to have a clinical
focus in their training, a fo cus that i s not required for a b asic scien ce
PhD p redoctoral trainee. Further, it is impo rtant t hat the training
plan does not only focus o n the technic al aspects of the training but
also includes information for the intellectual training a nd training in
nontechnical aspects that a person needs to bec ome a success ful
investigator. Therefor e, one of the most common mistakes a sponsor
makes is to provide a generic training plan that gives minimal
details and focuses solely o n how t he research will provide good
technical training.
In general, the training plan can be broken down into several differ-
ent topics: Formal education and examinations (predoctoral students
59Who’s the Boss?—Sponsor, Collaborators, and Consultants
only), technical training, research and professional training, seminars
and colloquia, and monitoring of the applicants progress.
Formal education and examinations: While not essential informa-
tion for the training plan of a pre docto ral student, a very short
description of the predoctoral or MD/PhD educational program
(classestaken,examinationsthestudent will take, etc.) provides
information that will indicate a supportive educational environment
exists for the training of the student. This information will be covered
in extensive detail in the Institutional Envir onment and Commitmen t
to Training section (co vered in Chapter 6), but including a n abbre vi-
ated version in this section along with the focus on how the formal
education w ill contribute to their overall training is always bene ficial .
If this informati on is in clude d, it is important that the Sponsor
explicitly state that a more extensive description of the overall educa-
tional program can be found in the Institutional Environment and
Commitment to Training.
Technical training: Within the training plan the most obvious infor-
mation to include is a discussion of the technical skills that will be
learned during the training period. Many times the first and only thing
that a sponsor will discuss in the training plan is the type and extent of
technical training that the applicant will receive. However, while tech-
nical training is important, it is not necessary for the sponsor to go
into too much exhaustive detail about the techniques that the applicant
will learn. The reviewers will read the Research Training Plan, in
which the scientific project is described, and will be able to evaluate
for themselves exactly what technical training the applicant will
receive. What is important for the sponsor to discuss in this section is
how the techniques the applicant will learn during the present training
period are distinct, different, and new from their previous research
experiences and how this new technical training will prepare them for
their subsequent career.
Research an d profe ssional training: Often times s ponsors neglect to
discusstheaspectsoftrainingthatIrefertoasthe“intangibles.”
Unlike the technical train ing or educational t rainin g, wher e you
have a “tangible” outcome in the form of grades or the acquisition
of expertise in a new procedure, the re are components of training
and professional development that do not have such specifically tan-
gible o utcomes. These intangibles take many differ ent forms an d will
60 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
be different for t he different stage s of training and for each individ-
ual trainee. A few examples are illustrated through the following
questions and suggestions for how t o address such “intangibles” in
your training plan: (1) Ho w wil l you teach the ap plicant to develop a
sound project, experimental desig n, and the an alysis of the ir resul ts?
“I will initi ally discuss the goal of the experiment with the applicant
after which I will allow them to design and perform the experiment
on their own. I wil l provide them sufficient time to a nalyze their
results at which time we wi ll meet, the ap plicant will pre sent t he
resultsandtheirideas,andIwillplayDevil’sAdvocatetodiscuss
the pros and cons of what they did.” (2) How will you mentor the
applicant in the presentati on of their w ork? “Iwilldiscussthepur-
pose of each section of the manuscript/grant with the applicant,
allow the m to p repare the first draft, after which I will work clos ely
with them to direct them in th e deve lopment of a sol id doc ument.”
(3) How often will you meet with them? “Because of my smaller lab-
oratorysize,Icangivetheapplicantmorepersonaltimeandassuch
I meet with them on a daily basis. In ad dition, we hold weekly/
monthly/periodic lab meetings in which all members of the lab pres-
ent their work.”
In addition to these more direct aspects of training in the experi-
mental and presentational aspects of an independent scientist, it is also
important to discuss the educational and teaching components of an
academic career. For example, as an academic scientist, the applicant
will be required to mentor students of their own and teach in classes,
both of which are things that must be learned. Therefore, it is impor-
tant for the sponsor to discuss within the training plan the opportu-
nities that will be provided for the applicant to gain these types of
experiences. These opportunities can take the form of directing a sum-
mer or rotation student, assisting in educational outreach programs, or
serving as a teaching assistant for a class.
Many times, a topic that is almost never discussed in the training
plan, but is important for the ability of the applicant to establish colla-
borations and to proceed to the next stage of their career, is how will
you, the sponsor, provide opportunities for the applicant to network,
prepare the applicant for the next stage of their career, and even assist
them in obtaining their next scientific position? Several examples for
how to address this issue are as follows: “I will frequently discuss the
61Who’s the Boss?—Sponsor, Collaborators, and Consultants
importance of publishing in good quality journals with the applicant”;
“The students and postdoctoral fellows are given the opportunity to
meet with invited seminar speakers providing them with valuable net-
working skills”; and “As the applicant nears the completion of their
training I will talk about their research interests and assist them in
making contacts with colleagues to obtain an appropriate position.”
What is most important though is in the discussion of these intangibles
it is essential for the sponsor to realize that this must be an individual-
ized plan that is tailor-made for the applicant. Too many times an
application comes to study section in which it is obvious to the
reviewers that a “cut-and-paste” approach has been taken. This pre-
sents a poor picture on the sponsor and is an indication of the level of
interaction and/or attention and quality of training the applicant will
receive, or not receive, from the sponsor.
Seminars and journal clubs: It is important that the sponsor discuss
the opportunities that will be made available for the applicant to
attend seminars on a regular basis. Further, you should mention, if it
is the case, that the applicant will have a chance to meet the visiting
speaker, which provides invaluable experience in meeting with collea-
gues and networking to create new connections, collaborators, and/or
job possibilities. The reviewers also want to see that the applicant will
be given a chance to present their work at departmental, institutional,
or regional seminars because it is in these “friendly” environments
where they will learn how to effectively present and defend their work.
Finally, many reviewers feel that a training plan is not complete with-
out the inclusion of a statement about participation in journal clubs. It
is in this educational environment that students and postdoctoral fel-
lows learn how to critically read and evaluate the literature, thereby
enhancing their own development and ability to write an effective
manuscript.
Monitoring of the applicant’s progress: An effective training plan
not only needs to contain details of the exact training the applicant
will receive but also needs some indication of how the sponsor will
monitor the progress of the applicant. This section does not need to be
extensive. For example, for predoctoral students this could take several
forms, including daily or regular interactions between the sponsor and
the applicant, regular thesis committee meetings, or departmental over-
sight committees. For postdoctoral trainees, in addition to the regular
62 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
meetings with the sponsor, this could include the formation of an advi-
sory committee that meets on a regular basis.
Inclusion of opportunities to interact with clinicians and/or clinical
research (MD/PhD predoctoral students only): The purpose of the
MD/PhD training program is to develop the individual into a clinician
scientist. This means that in addition to acquiring the basic knowledge
and skills needed as a clinician, they also require the skills needed for
them to develop and run a viable research program. Many times these
trainees have an interest in progressing on to a career in translational
science, which marries their two skill sets: Clinical knowledge and
basic science abilities. Therefore, it is often beneficial that the Sponsor
include information on how the MD/PhD trainee will be exposed to
clinical situations or how their thesis project will contain clinical
aspects to complement the basic science research. In this way, they will
not only get training in how to develop a project and conduct basic sci-
ence research (the focus of the PhD portion of their degree), but they
will also obtain training in how to conduct translational research and
correlate basic science findings to clinical situations.
Developing an independent project (postdoctoral fellows only): Every
single member of the study section has experienced firsthand the diffi-
culties involved with establishing their first laboratory. They know
how critical it is that a postdoctoral trainee has a project that they
have developed and are able to take with them to establish their first
independent laboratory. Therefore, it is essential that the sponsor
include a statement in the Training Plan for a postdoctoral fellow that
they will allow the applicant to develop an independent project during
their training period to take with them to establish their first lab.
Many times the absence of any description that the applicant will be
provided with this courtesy, thereby allowing them to “hit the ground
running,” negatively affects the quality of the training plan and subse-
quently the overall impact score of an application. It must be noted
here that this statement must be mirrored by a similar statement by
the applicant in the Selection of Sponsor and Institute section
(Chapter 3).
4.2.4 Environment and Resear ch Facilities
The details included within this section of the sponsor’s information
are also included as a separate section of the application (to be dis-
cussed in more depth in Chapter 6). However, even though including
63Who’s the Boss?—Sponsor, Collaborators, and Consultants
this information in this section may seem to be redundant, the infor-
mation cannot be abbreviated here. In this component provide infor-
mation about all aspects of the laboratory, the department, the
institute, and the region (if appropriate) that will be supportive of suc-
cess. In addition to specifics about the laboratory size, proximity to the
sponsor’s office, etc., this section needs to include information about
the proximity to other institutes, any groups that foster interactions/
seminars/training, and the intellectual environment of the laboratory
and/or department. In the Research Facilities, include descriptions of
all required laboratory space, equipment, and any core or animal facil-
ities that are essential to the success of the project and the training.
4.2.5 Number of Fellows/Trainees to be Supervised During the
Fellowship
This section of the Sponsor’s information is short, sweet, and to the
point. Simply list the total number of postdoctoral fellows, predoctoral
students, and/or undergraduate students that will be present in the lab-
oratory, and therefore vying for the sponsor ’s time and attention, dur-
ing the fellowship period. Also mention any predicted summer
students, rotating students or the potential for accepting additional stu-
dents and/or postdoctoral fellows throughout the course of the entire
training period. Of course it is not possible for a sponsor to know
exactly how many trainees will be passing through the lab for the
entire training period; however, it is possible to make a prediction
based on past history. For example: “During the period in which the
applicant will be in the lab I expect to accept a summer intern each
year, two to three rotating students each year, with the potential for
one of these rotating students to join the lab.”
4.2.6 Applicant’s Qualificatio ns and Potential for a Research
Career
Simply put, this is the sponsor’s letter of recommendation for the
applicant. In this section, the sponsor states their opinion of the appli-
cant’s capabilities in the tangible scientific and technical aspects and in
the “intangible” aspects described above. If the applicant had poor
grades as an undergraduate or graduate student, the sponsor can refute
this as an issue by telling the reviewers how outstanding they are now
and how those grades are not indicative of the applicant’s future
potential as an independent scientist. If the applicant had personal
issues that affected their previous performance or professional issues
64 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
with a previous advisor that delayed publications, this should be
addressed in this section, too. Most importantly, the sponsor must
explicitly state their opinion on the potential the applicant has for a
successful independent research career.
4.3 IS IT NECESSARY TO INCLUDE A
COSPONSOR—YES OR NO?
Probably one of the hardest things to determine, for applicants, spon-
sors, and even reviewers, is whether the inclusion of a cosponsor is nec-
essary. The purpose of the cosponsor is to contribute qualities to the
overall training that may be perceived to be deficient in the primary
sponsor. These qualities can include scientific or technical expertise,
proven expertise in training, a solid track record of publishing and/or
obtaining funding, or all of the above. In some instances the decision
to include a cosponsor is not in question. For example, assume the pri-
mary sponsor is a junior faculty member who has been in their inde-
pendent career for only a few years. Because of this junior status, they
have yet to successfully train any predoctoral students or postdoctoral
fellows, there are no publications from their independent laboratory,
and they have a lab with four predoctoral trainees, two postdoctoral
trainees, and one lab technician. The lab is still funded from start-up
money that will expire in 2 years and this person has yet to obtain sub-
stantial extramural funding. From this information it is obvious that
this sponsor does not have the requisite training experience, they do
not have guaranteed funding for the entire duration of the training
period, and in an effort to get as much work done as possible, have
taken on a workforce that even a seasoned investigator may have diffi-
culty managing. In this example, a cosponsor who is senior in rank,
has successfully trained a significant number of individuals ( . 10), has
a solid history of funding and publications, and has scientific expertise
in the field of the research is required. In contrast, assume the sponsor
has been in their independent career for 20 years, has trained 15 pre-
doctoral students and 10 postdoctoral students, the sponsor has had
consistent funding for 15 years and they have dedicated funding for
another 5, and they have an extensive list of publications in highly
respected journals. This person does not need a cosponsor. However,
these cases are usually the exception and not the norm.
65Who’s the Boss?—Sponsor, Collaborators, and Consultants
As a guideline to determine whether you need to include a cospon-
sor, ask yourself the following questions:
1. Does my sponsor have independent, extramural funding?
2. Does the sponsor’s funding cover the entire duration of the training
period of the applicant?
3. How many students has the sponsor trained and where have these
trainees gone?
4. What is the sponsor’s productivity as an independent researcher?
These questions will address many of the issues that reviewers con-
sider when determining the qualifications of the sponsor. If your
answer to most if not all of these questions is negative (as in the exam-
ple given above for the junior investigator), then you should strongly
consider including a cosponsor. However, everyone’s situation is not
necessarily as straightforward as the illustrations given above. For
example, a sponsor in mid-career (i.e., Associate Professor in their
independent career for 10 years) has funding to cover the duration of
the training period of the applicant. However, the sponsor works at a
smaller institute and as such has only trained four predoctoral students
and one postdoctoral fellow in the 10-year time period. These trainees
have gone on to productive careers at such institutes as Harvard and
tenure-track faculty at prominent academic institutions. Despite having
a small lab size (three individuals maximum at a time) the sponsor has
been highly productive publishing an average of one to three manu-
scripts a year based on research produced in their lab in solid journals
for the last 7 years. While on first look it would seem that this sponsor
would not require a cosponsor, the reviewers considered the minimal
number of students trained as a negative (despite the quality of the
training they received) and deemed it necessary for the inclusion of a
cosponsor.
Finally, while issues of funding levels and funding duration are very
obvious to consider, the issue of training history and productivity are
definitely a point of differing opinions among reviewers. In one case, a
sponsor may have trained eight predoctoral students and three post-
doctoral fellows to outstanding positions in a 9-year career. To one
reviewer this may be an exceptional training history, both in quantity
and quality. To another reviewer this would be considered a moderate
training history and as such score the sponsor lower. Along these same
lines, consider the example given above in which the sponsor published
66 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
one to three manuscripts a year from a small lab. Some reviewers
would not necessarily consider this to be a highly productive lab while
others would factor in the number of publications and the small labo-
ratory size and deem it to be very productive.
If you find yourself in a situation where you fulfill some of the
unstated requirements of what many reviewers consider being a
“good” sponsor but not all of them, consider which of the require-
ments you do fulfill. While reviewers tend to place a heavier emphasis
on funding and training history, ultimately it boils down to natural
bias in the study section (see Chapter 2). If you find yourself in a
“gray area” for a first submission of your application and fulfill some,
but not all of these elements, try to sell yourself as a mentor, play up
the positives and alleviate the potential concerns the reviewers may
have about the negatives, and do not include a cosponsor. If the
reviewers then feel that, despite your qualifications in some areas, you
still require a cosponsor, include one in the resubmission.
4.3.1 Selection of a Cosponsor
Special care and attention must be made when selecting your
cosponsor. In addition to complementing the perceived deficiencies
of the sponsor, the cos ponsor must provide expertise that is specific
to the research project and training of the applicant. In other
words, you should no t select a cosp onsor simply because they have
funding or simply because they have trained a large number of peo-
ple. It is essential that the cosponsor be integral to the training of
the applicant, both scientifically and professionally, and to also
serve as a “men tor” to the primary sponsor. Further , it is important
that the contributions of the cosponsor be seamlessly incorporated
into the entire application. Therefore, the applicant mus t provide a
description of why they selected this cosponso r (in the Selection of
Sponsor and Institute section) and exactly how the cospon sor will
contribute to their goals (in the Goals for Fellowship and Career
section).
The sponsor must clearly define exactly what the role of the cospon-
sor will be and the expertise that the cosponsor has that makes them
ideal for training the applicant (in the Training Plan section of the
Sponsor’s Information). For example, describe how the cosponsor will
serve on the applicant’s thesis or advisory committee; discuss the
67Who’s the Boss?—Sponsor, Collaborators, and Consultants
frequency and forum in which the cosponsor will meet with the student
to discuss data and experimental design; and describe any role the
cosponsor will play in training of the “intangibles” like manuscript
and/or grant preparation and networking. If the applicant is an MD/
PhD trainee, it is often beneficial to include a clinician scientist as the
cosponsor and have that person be integrally involved with the
research project.
Finally, in the Biosketch, which is included along with the
Biosketches of the applicant and sponsor, the cosponsor must discuss
in the Personal Statement their commitment to mentoring and exactly
how they will contribute to the training of the applicant. In addition,
the details about the cosponsor’s funding status, training history, and
number of trainees/fellows to be supervised during the fellowship must
also be included alongside this same information from the sponsor.
Remember, it is essential that the inclusion of the cosponsor is integral
in each and every section of the application, which therefore provides
the perception that they are a cohesive and important part of the
training experience.
68 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant

CHAPTER
5
5
Blind Them With Science—The Research
Training Plan
The third major component used to evaluate a Ruth L. Kirschstein
training grant is the Research Training Plan, in which you present the
scientific project that will be undertaken during the training period.
Unlike the R-series of grants, which focus entirely on the quality of the
science and the overall experimental design of the project, the
Research Training Plan in the Ruth L. Kirschstein training grants
focuses more on how the quality of the science, the significance of the
project, and logic of the experimental design contribute to the overall
training potential. This subtle shift in focus does not mean that less
care can, or should, be given to designing the project. A poorly laid
out research plan indicates poor mentoring by the sponsor in preparing
the application, which indicates a poor potential for training.
However, this subtle shift in focus does mean that some things com-
monly viewed as “fishing expeditions” (such as large-scale genomics
screens) are tolerated more in the Research Training Plan than they
would be in the R-series of research grants. Despite this “ increased”
tolerance, these so-called “fishing expeditions” still must be justified by
preliminary data or literature evidence and provide evidence that
clearly demonstrate a significant contribution to the overall training.
Further, although innovation is not explicitly required for the
Research Training Plan, the use of what are considered “standard”
procedures may detract from the perception of the training potential,
unless these “standard” procedures provide the applicant with training
in a new field, discipline, or technology.
The Research Training Plan contains all of the components that are
present in any standard National Institutes of Health (NIH) research
grant except for the inclusion of an Innovation section. In general,
innovation is not a consideration when evaluating the Research
A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant. DOI: https://doi.org/10.1016/B978-0-12-815336-9.00005-3
© 2018 Elsevier Inc. All rights reserved.
Training Plan. Overall the science portion can be broken down into
the following sections:
• Specific Aims (1 page)
• Research Strategy (6 pages)
• Significance
• Approach, in which each Aim is broken down into the following
sections:
• Rationale
• Preliminary Data
• Experimental Design
• Expected Outcomes
• Potential Problems and Alternatives.
The following descriptions are recommendations on how to con-
struct each of the sections for the Research Training Plan. These
recommendations derive from a basic understanding of the purpose
that each section and subsection plays in the overall grant. The struc-
ture and key phrases that are described in the examples below are
intended to help focus the writer on the purpose of each section, which
should allow you to impart clarity to the reader. It is important to
note that the examples that follow are simply recommendations and
not intended to be the “perfect” format for writing an exceptional
grant. Nor are these recommendations intended to serve as the only
way to write a clear, focused, and detailed Research Training Plan.
Instead, the following discussion is meant to provide an understanding
of the purpose that each section of the Research Training Plan plays in
the overall presentation of your project, and to provide key phrases
and ideas to help direct your writing while you develop your own
writing style.
5.1 SPECIFIC AIMS (1 PAGE)
In the pre-Internet, pre-electronic submission days, the only reviewers
that saw the entire grant package for any individual application were
the three assigned reviewers of that grant. All of the other study sec-
tion members received the Project Summary (Abstract) and the
Specific Aims page. Therefore, the Specific Aims was the most impor-
tant part of a science-oriented grant because this was the only part of
the application that every member of the study section would have
70 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
direct access to while discussing the application. Since the advent of
the Internet and electronic submission, all study section members,
regardless of whether they were assigned the application for full review
or not, have immediate access to every single grant under consider-
ation in their study section. Therefore, during study section, instead of
simply having the Specific Aims page, each reviewer can quickly and
easily pull up an application being discussed to read individual sec-
tions, scan the entire document, or just read the Specific Aims or
Project Summary. Although the easy access to the entire application in
some ways minimizes the original importance of the Specific Aims
page, it does not alter the fact that great care must be taken when con-
structing this section as it serves as a one-page overview of the entire
project.
Because the Specific Aims page serves as an overall summary of the
scientific project, it must contain all of the elements of the entire
Research Training Plan, which includes background of the field, identi-
fication of the gap in knowledge, significance of the project, a state-
ment of your hypothesis, literature and preliminary data evidence to
support the hypothesis, explicit aims that will address this hypothesis,
and how your results will impact the field. Conceptually, the Specific
Aims page can be broken down into four sections, which may visually
appear as four distinct paragraphs.
Paragraph #1: Conceptually, the first paragraph of the Specific
Aims serves as the background and significance. You need to establish
the importance of the disease and or scientific question that you are
investigating, which is usually established by utilizing statistics of dis-
ease mortality or morbidity. Once you establish the health relevance,
provide the reader with enough evidence to identify a gap of knowl-
edge in the field. Once you’ve identified this gap in knowledge, state
why it is a problem with advancing of the field, which may include
developing effective or novel treatments for the disease state under
study. For example: “Although some of the molecular mechanisms
regulating the biological activities of the key factors are known, at
present the exact role of posttranslational modifications, particularly
phosphorylation, in regulating both proteins has yet to be elucidated.
The absence of this knowledge will greatly impact the ability to
develop novel therapies to treat the disease.” Then conclude this para-
graph by explicitly stating how the long-term goals of the lab or the
71Blind Them With Science—The Research Training Plan
explicit goals of your project will address this gap and thereby advance
the field: “Therefore, it is the long-term goal of this lab to understand
how phosphorylation of the key factors regulate their biological activ-
ity, how this regulation contributes to normal development, and how
this regulation is altered to contribute to the development of the
disease.”
Paragraph #2: This paragraph is where you describe the objectives
of the project and state the central hypothesis of your proposed
research. For example: “In keeping with the long-term goals of the lab
the central hypothesis of this project is....” Follow this statement with
a detailed and explicit description of your hypothesis: “...differences
in the phosphorylation of the key factors throughout early differentia-
tion contribute to alterations in gene expression, thereby contributing
to the development of disease phenotypes.” Reviewers like to see what
is commonly termed “hypothesis-driven research,” in which there is a
very clear and direct hypothesis that is driving the overall project.
Many times a Research Training Plan will suffer because the applicant
does not formulate an explicit hypothesis or central driving question or
this hypothesis is stated in broad generalities and not detailed specifics.
After the statement of hypothesis, provide a description of the liter-
ature evidence that supports this hypothesis: “This hypothesis was for-
mulated from the following literature evidence...” with a concise, yet
detailed description, properly referenced, of the literature evidence that
supports your hypothesis. In addition to literature evidence, the
reviewers also like to see preliminary data that not only supports the
hypothesis but also supports the feasibility of the proposed studies.
Therefore, state: “In addition to this literature evidence I present pre-
liminary data that further supports the idea that (or provides feasibility
for the studies)...” again with a concise, yet detailed description of the
data you will present in the Research Training Plan. Finally, wrap up
this paragraph by leading into the statement of your specific aims:
“We will test our central hypothesis through the following two/three
specific aims.”
Alternatively, many people start this second paragraph with a
description of the literature evidence that they used to develop their
central hypothesis. This statement is then followed by a description of
the preliminary data that supports the hypothesis and/or the feasibility
of the studies. Finally, tie these two different lines of supportive logic
72 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
together to lead into the statement of your central hypothesis:
“Therefore, taken together, this information allows me to propose the
central hypothesis that....” As with the example above, the statement
of the hypothesis leads into the statement of the Specific Aims as
described above. It is important to note that bold and italic fonts are
purposefully used to highlight the statement of the hypothesis. The use
of a different style draws the reader’s eye to those words and makes it
easy for the reviewer to see that you have a very defined, clearly stated
hypothesis driving your work.
Paragraph / section #3: Explicitly state your specific aims in bold
letters. This is a 23 year project so two to three aims are appropriate.
In some cases, a brief one- to two-sentence description of the impor-
tance of the aim and the methods that will be used to test the aim can
be included:
Specific Aim 1: To examine the role of the phosphorylation of key
factors in development and as a contributor to the pathology of the dis-
ease. We will use the physiologically relevant primary cells or disease-
derived cell lines stably expressing the key factor or mutants in which the
identified sites of phosphorylation are mutated to phospho-incompetent
or phospho-mimetic amino acids. We will determine how phosphoryla-
tion at these sites contributes to normal development and the
development of disease by examining cellular functions, such as growth,
migration, differentiation, and by performing an unbiased survey to ana-
lyze changes in the transcriptome profiles during early myogenesis.
When constructing the specific aims for your project, great care
needs to be given to ensure that the aims you are proposing are interre-
lated but not interdependent. What this means is that you want to have
all aims contribute to addressing the central hypothesis of your project.
However, you don’t want the feasibility of one aim to be directly
dependent on the success of a prior aim. For example, suppose you
propose in Aim 1 to identify and characterize the sites of phosphoryla-
tion on a transcription factor. Then in Aim 2 you propose to generate
explicit mutants that target the identified sites in order to determine
the role they play in biological functions. In this example, although
these aims are obviously interrelated, the feasibility of Aim 2 directly
depends on the ability of being able to identify the sites of phosphory-
lation. If you are unable to identify these sites in Aim 1, then Aim 2 is
not possible and half of your project is a bust. In contrast, assume you
73Blind Them With Science—The Research Training Plan
have preliminary data that identifies the only sites of phosphorylation
on your protein of interest. Given this knowledge you propose in
Aim 1 to determine the effects of these mutations on biological events
(e.g., proliferation and differentiation, etc.) and in Aim 2 you propose
to determine the effects of these mutants on the molecular activities
(e.g., DNA binding, transcriptional activity, expression of target genes,
etc.). In this illustration, both aims focus on the effects of the identified
sites of phosphorylation have on the molecular and biological func-
tions of the protein. However, the success of the second aim is not
dependent on the success of a previous aim.
Paragraph #4: Finally, you want to provide a summary for the
reader, which is the conceptual basis of this final paragraph. Tell the
reviewer what each aim will achieve and how the successful completion
of this aim will provide information that will advance the field: “The
research accomplished in Specific Aim 1 will provide an understanding
for how changes in the phosphorylation of key factors contributes to
both normal differentiation and the development of the disease.
Completion of Specific Aim 2 will provide an understanding of the
role that phosphorylation plays in regulating the transcriptional and
biological activities of the key factors.” Also, it is important to tell the
reviewer exactly how these results could be used for future studies or
what you visualize the long-term impact of this project to be.
“Therefore, by understanding the mechanism by which phosphoryla-
tion of this protein affects tumor development, we will be able to
identify novel molecular targets that can be used for the creation of
new pharmaceutical therapies for the treatment of this cancer.”
Although this scientific program is part of a training plan, the
reviewers like to see that you are able to think past the present work
and that you understand the potential impact of your results. Many
times a grant will not be considered as strong as it possibly could be
because the applicant did not adequately demonstrate that they under-
stand the implications of their work to the larger field and future
experiments.
It is advisable to include a statement of the contributions that the
proposed research will make to the training potential of the individual:
“The applicant, an MD/PhD candidate, will develop the necessary
technical and critical thinking skills, including the development and
analysis of behavioral and molecular studies, to ensure success in a
translational research career under the mentorship of the sponsor, an
74 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
established researcher and MD/PhD scientist in the field of research.”
Although not essential for a successful application, this final statement
summarizes for the reader exactly how the applicant will obtain key
training for them to obtain their career goals.
5.2 SIGNIFICANCE ( 0.5 PAGES)
In January 2010, the NIH implemented a new, shorter format for
grant submissions. This shorter format modified subsections of the
grant to provide a different focus than the original, longer form. One
of the sections that changed was switching from a “Background and
Significance” section to a section entitled “Significance.” In the origi-
nal format the Background and Significance consisted of an extensive,
multipage review of the literature that described the field in which the
proposed research was being conducted. This section also contained a
statement, within the context of the large literature review, of why the
research described in the proposal was significant. In the new format,
however, the purpose of the Significance section (note the lack of the
word Background in the section heading) is to focus entirely on the
just that... the significance that the research presented in the Research
Training Plan has to impact the field of study. This section is not
intended to replace the Background and Significance section from the
old format! Therefore, the inclusion of a several page discussion of
background information is not required, and within the shorter format,
which for a Ruth L. Kirschstein NRSA training grant is only six pages
for the entire Research Strategy, the luxury of having such an extensive
discussion is not feasible.
The purpose of the “Significance” section is to explicitly state why
your work is significant in relation to your field of study and how the
results from the proposed project will impact the field. The absence of
the word “Background” in this new format is not meant to imply that
this section does not contain any background information.
Background literature is essential to provide the reader with enough
context about the field of study so that they can evaluate your interpre-
tation of how the proposed research is significant. In contrast, this
background information should encompass only a few sentences and
not several paragraphs, or even pages (as was required in the former
format). In many respects, this section will provide essentially the same
information found in the first paragraph of the Specific Aims page (see
75Blind Them With Science—The Research Training Plan

above), and in fact it is advisable to paraphrase the first paragraph of
the Specific Aims page to begin this section. However, you will provide
more detail in the Significance section in discussing the literature evi-
dence that creates the foundation of your proposed work and how
your work is essential for advancing the field.
Once you have provided enough evidence to create a solid back-
ground foundation, identify the gap in knowledge in the field:
“Despite this information, the mechanism by which X does Y to
contribute to disease progression is not yet known.” Once you have
identified the deficiency, explicitly state why this is a problem to
advance the field: “Without understanding the mechanism by which X
does Y, it will be difficult to develop novel therapies for the treatment
of the disease. ” Then explicitly state in
bold, underlined, italics:
“
Therefore, the contributions of the present proposal are significant
because it will be the first study to...” As in every other important
statement within the Research Training Plan (hypothesis, objective,
long-term goals, etc.), be explicit and detailed in your statement.
Again, the use of bold, underlined, italics draws the reader’s eye and
makes it easier for a potentially tired reviewer to see that you have
explicitly stated the significance of the work. Finally, as with the
Specific Aims, end the Significance section with a statement that
informs the reader exactly how the successful completion of the pro-
posed research will push the field forward and could be used for future
studies: “By understanding the mechanism by which X does Y we
will be able to identify new molecular targets to be used for the devel-
opment of novel pharmaceutical therapies for the treatment of the dis-
ease.” As with the Specific Aims, the reviewers like to see that you are
able to think past the present project to see the overall implications of
the work and that the project is not simply “research for research
sake.”
5.3 APPROACH ( 5.5 PAGES)
The old NIH grant format contained independent sections for
Background and Significance, Preliminary Studies, and Research
Design. The new, shorter format contains two sections: Significance
(which was discussed above) and Approach. Therefore, the back-
ground, preliminary data, and research design are all encompassed
within the new Approach section, in essence condensing nearly 8 10
76 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
pages of writing into an approximately 5-page space. This may at first
seem like an insurmountable task. However, the purpose of creating
the new format was to help facilitate the review process by decreasing
the amount of time a reviewer spent reading an application. Therefore,
the new format condenses the writing from a broad-spectrum docu-
ment to a more focused work that is meant to contain only informa-
tion that is absolutely essential to support and describe the proposed
project.
To condense and focus your writing, it is often beneficial to think
of the Approach section in terms of the Specific Aims. What this
means is that instead of providing background information and prelim-
inary data for the entire project, separate the Approach into parts that
correspond to the number of aims that you have proposed and then
provide the background information and preliminary data essential to
support that individual specific aim. Within each section, or specific
aim, organize the writing to include three subsections entitled
Rationale, Preliminary Data, and Experimental Design. For example,
if you are proposing a project with two specific aims, the Approach
will be separated into two parts, one part for each aim. The title of
each section will be the title of the Specific Aim, exactly as written on
the Specific Aims page, followed by subheadings for each part:
Specific Aim 1: To examine the role of the phosphorylation of key factors
in development and as a contributor to the pathology of the disease.
Rationale: This section includes the literature evidence that supports
the objective and working hypothesis for this aim.
Preliminary Data: This section includes the preliminary data
obtained by the applicant that supports the hypothesis and the feasi-
bility of the project for this aim.
Experimental Design: This section describes the experiments and anal-
yses that you propose to address the working hypothesis for this aim.
This section also includes a description of the expected results, poten-
tial problems, and suggested alternatives should problems arise.
5.3.1 Rationale
About one paragraph in length, the Rationale provides background
that supports the working hypothesis for each individual aim.Itisin
77Blind Them With Science—The Research Training Plan

this section that you discuss the literature evidence that was used to
support the objective of the aim. Much of the same literature evidence
mentioned in the first paragraph of the Specific Aims page and in the
Significance section will be used in the Rationale. However, the most
detail is used here to describe the specifics of the literature. You want
to explicitly describe the results in the literature that you have used to
support your working hypothesis for this aim. You must provide
enough detail such that the reader understands the importance of the
results and how the results pertain to the overall objective for this par-
ticular aim. However, remember that you have very limited space, so
it is important that you be as direct and concise as you possibly can.
Once you have described the literature evidence, summarize what
you have just written by explicitly stating how this evidence supports
the hypothesis or the feasibility of the aim: “Taken together, this evi-
dence shows that....” After this, as with the Specific Aims and the
Significance, state what the gap in knowledge is as it relates to the aim
under discussion: “However, despite this knowledge, the mechanism by
which phosphorylation contributes to the molecular mechanisms of
disease pathology is not yet known.” Follow this statement with a
clear and explicit statement of the objective and working hypothesis
for this aim again written in
bold, underlined, italics: “ Therefore, the
objective of this aim is to test the working hypothesis that....”
5.3.2 Preliminary Data
In addition to solid literature support, reviewers almost always want to
see preliminary data that supports the hypothesis you have proposed.
Through the preliminary data you also give the reviewer confidence
that you are technically capable of performing the proposed research
and that your model system and/or hypothesis are valid and func-
tional. The validity and functionality of your model system are impor-
tant. Your training period, regardless of the granting mechanism (F30,
F31, or F32), will be about 23 years. Therefore, the reviewers do not
want to see a proposed project in which you will spend a majority of
your time developing or validating a novel model system. They want
to see an experimental model in place and know that you are capable
of using this model to obtain viable data to address a specific question.
After beginning this section by stating; “In addition to published
reports, my preliminary data supports the hypothesis that...”
78 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
systematically present your preliminary data. If you include unpub-
lished data from another member of the lab, it is essential that you
identify this fact. Confusion among the reviewers regarding who actu-
ally did the work presented in the Research Training Plan will affect
the score of this section and may affect the overall impact score. It is
acceptable to include a short statement in the figure legend for a par-
ticular piece of data such as: “This data was generated by....”
Alternatively, it is also acceptable to include a clarifying phrase in the
body of the text when introducing the data in question: “Using data
generated by....”
In addition to being clear about who generated the data, it is critical
that the inclusion of the figures be presented in an ordered, logical, and
neat manner. Many times an application will be submitted in which the
figures appear to have been haphazardly imported into the document
with no apparent logic for where and how the figures were placed (see
Fig. 5.1). Visually this gives the impression of sloppy work and results
in a poor first impression, which may suggest to the reviewer that the
lack of attention to detail in putting together the application may be
indicative of the type of science the applicant will perform or the train-
ing that they will receive. Instead, align each figure of data with the
paragraph that describes the data, insure that the figures align to the
edge of the text, and make sure that there is a consistent distribution of
the figures (i.e., all figures align on one side of the page or there is an
alteration of alignment) (see
Fig. 5.2). Remember, the first impression a
reviewer will have is the visual impression. The appearance of sloppy
work or a document with no “white space” to provide rest for the eye
may predispose a potentially tired reviewer to a negative impression
before they even begin to read your science.
When discussing your data, it is recommended to use one paragraph
for each experiment, point, or conclusion that you are presenting. This
physical and visual separation of experiments allows the reviewer to
focus on one thought and idea at a time and gives a visual impression
of discrete conceptual units. Also, by devoting one paragraph to each
experiment it is easy to import the figure illustrating this data so that it
is embedded within the paragraph in which the data is being discussed.
The easiest way to import your data into the text is through the utiliza-
tion of the Text Box tool in Microsoft Word. Simply insert a text box
where you would like the figure to appear, copy your data into the
text box, and then type the figure number and figure legend
79Blind Them With Science—The Research Training Plan
immediately below the data but within the text box. The inclusion of
the figure legend within the text box along with the data it is describing
keeps the two items together as a single “unit”, which greatly facilitates
their positioning and movement within the document. Once inserted
simply format the text box so that the body of the text wraps around
the box.
Figure 5.1 Illustration of poor presentation of preliminary data.
80 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant

In addition, you need to make the basic assumption that the person
reading your application will know nothing about your field of
research and therefore may not implicitly understand why you per-
formed each experiment or why the results are important to support
your hypothesis. It is more than likely that the reviewer reading your
grant may be familiar with the techniques you are using, but they will
Figure 5.2 Illustration of organized presentation of preliminary data.
81Blind Them With Science—The Research Training Plan
know very little about the field in which you are working. Therefore,
you must be explicit, detailed, but yet concise as you describe the
thought processes underlying each experiment. In essence, walk the
reviewer through each experiment starting with why the experiment
was performed, how the experiment was performed, what the results
looked like, and the conclusions drawn from these results.
The following rubric can serve as a model for the construction of
each individual paragraph of the Preliminary Data section. First, tell
the reader exactly what the purpose of the experiment is, how it
derived from literature evidence or unpublished data, and how it
relates to the hypothesis or model system. “Literature evidence sug-
gests that phosphorylation of the transcription factor is important for
regulating differentiation. However, to date, no experiments have been
performed to test this idea. Therefore, to determine how phosphoryla-
tion at specific sites affects the functions of the transcription factor we
tested the ability of different phospho-mutants to alter DNA binding.”
Once you have established the reason for performing the experiment,
provide them with just enough information, usually one to three
sentences, to understand how the experiment was performed. This
description does not need to include minute details, such as buffers
used or concentrations of reagents, but should contain broader strokes
that include the experimental system used, the read out that provided
the data, and how the data was analyzed.
After discussing the experimental system you next want to describe
the results of the experiment. Do not assume that the reviewer will
understand or be able to interpret the data simply by looking at your
figure! Too many times an applicant will simply write “As evident in
Figure X, treatment of cells with the drug inhibits differentiation,”
without providing an explanation of what the figure is showing, what
the control is, what differentiation of this cell type looks like, etc. As
stated repeatedly, the reviewer will most likely not be versed in your
field of research, let alone be able to interpret data without at least a
minimal explanation. Making the assumption that the reader may
have an expertise that they might not truly have will only frustrate and
anger your reviewer. Therefore, be sure to describe the data to the
reader so they can make an intelligent evaluation of the data for them-
selves. Also, it is important to point out exactly what it is about the
results that you want the reviewer to focus on. For example, “We
observed the elongation of cells with fusion into multinucleated
82 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant

myotubes, which was confirmed by quantification of the percentage of
nuclei present in multinucleated myotubes (Figure X). Further, the
presence of the phospho-mutant inhibited differentiation, as evidenced
by a decrease in elongation and percentage of multinucleated cells rela-
tive to cells expressing the wild-type transcription factor.” In general, it
is not a good strategy to assume the reader will see exactly what you
see in the data or even if they do see it that they will agree with your
conclusion if the data is not fully explained to them.
Finally, spell out your conclusions from the experiment and
describe why these conclusions are important to support your hypothe-
sis for the aim or to provide feasibility for the experimental model.
“This data demonstrates that the ectopic expression of the phospho-
mutant inhibits differentiation, supporting the idea that the
nonphosphorylated form is essential for differentiation. Therefore, this
conclusion supports our hypothesis that....” If the data provides feasi-
bility or validity of the new experimental model system, state this fact
as follows: “ This data demonstrates that we have all of the reagents
required for the successful completion of this Aim and that the model
system utilized for all experiments is valid to study our working
hypothesis.” Once you have discussed all of your preliminary data for
that specific aim, provide a summary statement to further highlight
how, when examined as a whole, the mass of data you presented sup-
ports the idea that your project has a high level of feasibility and the
hypothesis is sound. “Taken together, published reports combined with
our preliminary data demonstrate that the expression of the oncogenic
protein results in distinct morphological and biological effects on pri-
mary cell differentiation. These observations, which most likely result
from global changes in transcriptional regulation, provide solid evi-
dence to support the idea that the presence of the oncogenic protein is
capable of altering global transcriptional regulatory networks to result
in the observed changes in differentiation, proliferation, and cellular
movement.” As with other sections of the Research Strategy, the
concluding statements for each experiment, along with the overall sum-
mary are best written in
bold, underline, and italics to highlight them
for the reader.
5.3.3 Experimental Design
After establishing the feasibility of your hypothesis and the validity of
your experimental model through literature evidence and preliminary
83Blind Them With Science—The Research Training Plan
data, you next logically lay out the series of experiments that you will
use to address the working hypothesis of this Aim. As with the
Preliminary Data section, each individual experiment will be described
in its own paragraph or sentence with the experiments being numbered
in sequential order (i.e., Experiment #1, Experiment #2, etc.). Begin
each experiment with a descriptive title that tells the reader the purpose
of this experiment: “Experiment #1: Determining the effects of phos-
phorylation of the transcription factor on cellular proliferation.”
Follow this title with an introductory sentence to tell the reader how
this experiment fits into context of the larger scope of the aim:
“Literature evidence demonstrates that the transcription factor is
involved in multiple aspects of cellular functions, including prolifera-
tion. Our preliminary data demonstrates that phosphorylation of the
transcription factor contributes to some of these phenotypes.
Therefore, this experiment directly tests the role that phosphorylation
of the transcription factor plays in cellular proliferation.”
After placing the experiment in context, provide several sentences
detailing the experimental design itself. As with the Preliminary Data,
it is not necessary to provide the minute details of the experiment (i.e.,
buffer concentrations, reaction volumes, incubation times, etc.).
However, it is essential to provide significant details that will allow the
reader to evaluate the construction of the experiment and the analysis
of the results. This means you should detail the technique that you will
be using to perform the experiment. You also need to describe what
samples you will use within the experiment and why you are including
them, what are the positive and/or negative controls that will be
included, what are the time points that will be used (if appropriate),
exactly why are you choosing those time points, and what is the output
that you will use to determine the results. Finally, you must include a
description of how you will analyze the results, reproducibility, and
statistics: “To determine how the phospho-mutant alters cellular prolif-
eration, we will compare the results obtained with the mutant to those
of cells expressing the wild-type factor. We will perform all experi-
ments in triplicate and normalize values for the negative control of
cells not expressing either protein.” It is not necessary to discuss the
expected results from the analysis at this stage. This information will
be provided later in its own section (see below).
One question that arises in the construction of the Experimental
Design component of the Research Training Plan is whether a section
84 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
detailing the exact methods to be used should be included. In the old
grant format, where an applicant had fewer space constraints, it was
possible to dedicate a full page or even more describing the minutiae
of the experimental details in a discrete section dedicated to methods.
However, the new format does not allow for such usage of space. The
reviewers generally make the assumption that a trainee will be experi-
enced enough to know the details of an individual experiment or if
they don’t, that they will have the intellectual resources in the labora-
tory to troubleshoot and learn these details. The reviewers are inter-
ested in seeing the “bigger picture” of the experimental design, as
described above, and that the applicant understands why they are
doing the experiment, what are the essential samples to be used in the
experiment, what is the basic assay and read out for the assay, and
how will they analyze the results. Therefore, it is usually not recom-
mended to include a specific section of the Experimental Design dedi-
cated to a description of the methods.
5.3.4 Expected Results
To convince the reviewers that you will be capable of interpreting the
results of your experiments, you need to provide them with a descrip-
tion of what you expect your results to look like, and how you will
interpret them, should your hypothesis be correct. You want to include
brief descriptions of the expectations you have for all of the experi-
ments included in the Experimental Design. This section can be diffi-
cult to write given the simple fact that sometimes the reason you are
doing the experiment in the first place is to determine what will hap-
pen. In some cases, you might have preliminary data that will give you
very solid groundwork to predict what you will see: “Based on our pre-
liminary data in which the mutation of the transcription factor resulted
in an inhibition of the effect, we expect to observe a decrease in our
experimental output with our mutant when compared relative to the
wild-type control.” Sometimes, too, you just don’t know what you will
see and you have no preliminary data or literature evidence to allow
you to make an educated guess. However, you can supply the reader
with a hypothetical situation that is based on your hypothesis, being
very careful to explicitly tell the reader that it is just that... a hypothet-
ical situation: “At present, it is difficult to determine how mutation of
the transcription factor will affect cellular biology. However, assuming
the hypothetical situation in which loss of the site is essential for prolif-
eration, then we would expect to observe a decrease in proliferation
85Blind Them With Science—The Research Training Plan
rate of the mutant relative to the wild-type control.” In essence, you
want to prove to the reader that once you get the data and the results
from the experiments that you will know how to evaluate them and to
interpret them based on your hypothesis.
5.3.5 Potential Problems and Alternatives
Finally, the reviewers want to see that you, the applicant, are aware
that problems can, and most likely will exist in the project and that
you have alternative methods should you encounter these problems.
Remember, if successful, the federal government, through your tax dol-
lars, will be giving you upwards of $100,000$150,000 in total for
your training. They want assurance that if you run into problems that
derail your project that this money will not be wasted. Many Research
Training Plans suffer from the very simple fact that the applicant did
not include any description of potential problems and alternatives to
these problems. What is important is that you do not state that you
expect no problems! This is science. The people reading your applica-
tion are scientists, many of whom have been working in research for
years if not decades. They all know that research is fraught with pro-
blems both technical and intellectual. Therefore, the statement that
there should be no problems will be viewed for what it is... a naïve
statement. However, if there is a technique that you are using that is
standard practice in the lab in which you are working you can state
the following: “The techniques described in this aim are routinely per-
formed within the lab and as such are not expected to present any
major technical difficulties.” You must be sure to follow this statement
up by identifying some valid problems (e.g., transfection efficiencies
are inadequate and limits of detection are not feasible) and provide
descriptions of viable experimental alternatives to these problems.
Most importantly, unless you have solid evidence that supports
your hypothesis incontrovertibly, consider very hard the simple fact
that your hypothesis may be wrong. Hypotheses are developed specifi-
cally to be tested through experimentation in order to achieve an
answer. Part of your training in basic research is to learn that some-
times the answer to these questions are “no” and that your hypothesis
as originally constructed is incorrect. Therefore, you must provide the
reader with a solid description of what you intend to do should your
hypothesis be incorrect, either in part or in its entirety. Are there other
pathways that may be considered? Are there other explanations that
86 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
could lead to the same phenotype that could then be tested? Do you
have the ability to reconstruct a new hypothesis on alternative data
that can subsequently be tested? Let them see that if your hypothesis is
incorrect, that you know what to do, and that you have alternative
options or explanations to test so that the money given to you by the
federal government will not be wasted. You don’t want to undersell or
undermine your hypothesis, as that is the cornerstone of your project.
However, recognize the fact that hypotheses may not necessarily be
correct as constructed.
87Blind Them With Science—The Research Training Plan

CHAPTER
6
6
Last but Not Least: Institutional Environment,
Training Potential, and Other Scored Items
There are two remaining major criteria used to evaluate a Ruth L.
Kirschstein training grant: the Institutional Environment and the Training
Potential. Of these two, the Training Potential is primarily subjective while
the Institutional Environment, which is evaluated through three indepen-
dent sections Facilities and Resources, Equipment, and Institutional
Environment and Commitment to Training, is primarily objective. The
Institutional Environment has an explicit section in which the applicant
and sponsor describe the educational and scientific environment. In con-
trast, the quality of the Training Potential is determined through consider-
ation of all parts of the grant and how they combine to provide an overall
training experience. In addition to these last two explicitly scored criteria,
there are several other informational sections that do not receive an inde-
pendent or individual score but whose content contributes to the overall
score: Activities Planned, Vertebrate Animals, and Research Sharing Plan.
6.1 INSTITUTIONAL ENVIRONMENT
The Institutional Environment is probably the most objective of the
five main criteria used to evaluate the application, simply because
either the institute has the resources or it does not. The reviewers want
to see that the environment in which the training will occur has all of
the facilities, equipment, intellectual, and educational resources
required to provide the applicant with an optimal training experience.
This optimal experience includes easy access to the cores, facilities,
equipment, and resources needed to successfully perform the research.
In addition to providing explicit descriptions of the facilities and the
equipment available to the applicant, it is now required to include
an explicit description of the academic, educational, and intellectual
environment in which the training will be performed.
Combined, the information that describes the facilities, equipment,
resources, and environment is covered in several different places within
A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant. DOI: https://doi.org/10.1016/B978-0-12-815336-9.00006-5
© 2018 Elsevier Inc. All rights reserved.
the application. First, the applicant provides two distinct parts:
“Facilities and Resources” and “Equipment,” each of which is its own
self-contained document and is uploaded separately on the application
form. Second, the applicant is required to include an attachment
explicitly titled “Institutional Environment and Commitment to
Training,” which was recently deemed a required document for all
applications. Finally, the sponsor includes a description of the training
environment within the Sponsor and Cosponsor Information section.
In the context of the sponsor’s information, the descriptions may not
be as detailed, but will in many ways duplicate what is included in the
other two sections.
6.1.1 Facilities and Resources
In essence, the reviewers want to see that the applicant has all of the
required facilities and resources at their disposal to facilitate the research
and the intellectual training. Therefore, the description of the facilities is
not to be limited to the physical resources but also needs to discuss the
intellectual resources available that will truly enhance the training experi-
ence. It is easiest to present this information as explicit sections:
• Laboratory space: Describe in detail the laboratory space available.
Include a description of the square footage, the number of desks
and benches available, and how many people it can comfortably
accommodate. If you describe tissue culture experiments in your
Research Training Plan, provide a description of the tissue culture
facilities and if they are in a separate laboratory, describe the size of
this laboratory and the proximity to the main lab.
• Animal facilities: If you include animal work in your Research
Training Plan, it is essential that you provide a description of the
animal facilities at your institute. Tell the reviewers how many
rooms are available to the laboratory and the proximity of the ani-
mal facilities to the main laboratory. Describe environmental con-
trols (light/dark cycles, temperature, etc.) that the animals will be
housed in, especially if these environmental factors are essential to
experiments described in the Research Strategy.
• Office space: Provide a description of the sponsor’s office space,
including the square footage and the proximity of this office to the
laboratory. If the institute or department provides a graduate stu-
dent and/or postdoctoral office area, describe this area, include its
proximity to the laboratory, and mention how this “communal”
90 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
area facilitates interactions between students and postdoctoral
fellows.
• Computers: Describe the computers that are available in the labora-
tory and whether each person will have their own computer (desk-
top or laptop) or whether these computers will be shared. Also,
include a statement about what programs will be on the computers
that are essential for the work (Microsoft Word, PowerPoint, Excel,
graphing programs, etc.) and the connectivity to the Internet.
• Core facilities: If your Research Training Plan includes a technique
or series of experiments that is perceived to be specialized and there-
fore requires a specifically trained individual (e.g., genomics, bioin-
formatics, proteomics, etc.), then describe the core facility that will
aid you in these experiments. If a particular instrument is being used
in the core, describe that instrument (e.g., Illumina Genome Analyzer
2X with associated equipment for the cluster generation). Indicate,
too, who will operate the machine and the training they have.
• Other resources: These are resources that contribute to the intellec-
tual development and provide support services to make day-to-day
working possible.
• Intellectual and training environment: Provide information on any
research or seminar groups to which you belong. Describe any
interactions that your lab or department has with other labs or
departments in the institute, indicating how these interactions will
promote an exposure to different research disciplines. If your
institute is in close proximity to other research institutes then dis-
cuss this fact and describe any opportunities you will have to
interact with these places. Finally, if there are any organizations
within your institute that provide career development, describe
them since planning for your future career is a significant part of
your training. Although this last resource is useful to graduate
students, it is extremely important for inclusion as a resource for
postdoctoral trainees, since part of their training will be the tran-
sition to their first independent academic position.
• Administrative support: The support staff facilitates the day-to-
day workings of a laboratory or a department. Most likely the
business administrator assisted the applicant in putting together
the budget and the final application. Departmental coordinators
assist in organizing seminar series, reserving of rooms, etc. Briefly
describe the administrative support staff available and how they
will assist the applicant.
91Last but Not Least: Institutional Environment, Training Potential, and Other Scored Items
6.1.2 Equipment
As with the facilities, the reviewers need to see that you have all of the
necessary equipment to perform the experiments described in your
Research Training Plan. Very simply put, describe, in detail, the equip-
ment at your disposal. Do not simply state “the laboratory has four
PCR machines” but provide information on what make and model:
“The laboratory has four Applied Biosystems Geneamp 9700 PCR
machines.” If you are using incubators for samples that require two
different temperatures (e.g., bacteria vs. yeast) indicate that incubators
maintained at these temperatures are present. If you are performing
research that requires a specialized piece of equipment (e.g., immuno-
fluorescent imaging), describe the microscope that will be used for this
imaging and whether it is confocal or not. If there is equipment that
belongs to the department, state “The following major equipment is
present in the Department and will be available for the applicant’s
use.” Of note, the level of detail indicated above is not necessary when
describing standard items, such as refrigerators and freezers. However,
with these items it is important to indicate that there are such storage
areas available at a variety of different temperatures essential for stor-
ing different reagents and samples.
6.1.3 Institutional Environment and
Commitment to Training (2 pages)
In addition to seeing that the technical and intellectual resources are
available for the applicant, the reviewers need to see that the depart-
ment and/or institute have a commitment to the applicant’s success.
Previously, an independent document that supplemented the informa-
tion included in the Facilities, Equipment, and Sponsor/Cosponsor sec-
tion was not necessary. However, the National Institutes of Health
(NIH) now requires that an independent document be included in the
application that describes the institutional, departmental, and labora-
tory environment along with a description of the educational programs
for PhD and MD/PhD trainees (F30 and F31 applications). The inclu-
sion of this separate document does not preclude including this infor-
mation in the other places already described; however, this section
does allow the applicant to expand on what is written elsewhere in the
application to provide more detailed information.
Although much, if not all of the information included in this sec-
tion is desc ribed in other part s of t he ap pl ication, this newly requi red
92 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
section coalesces this inf ormation in to one defined place, mak ing it
easier for a reviewer to evaluate the instit uti onal environment. The
NIH guidelines are very specific about what information is to be
included here. It is nece ssary to discuss how the training will be
undertaken within a laboratory with a well-established research pro-
gram and a sponsor with a solid history of producing significant
work in a field or topic that is related to the p roposed project
described i n the Research Strategy. Informati on also needs to be
included that describes opportunities for intellectual interactions with
other laboratories and investigators. These int eraction s may include
courses that will be tak en, journal clubs, semi nars, present ation
requirements, and opportunities for attending s eminars and meetings
at regional institutions. It is also necessary t o discuss the resources
that are available for the applicant with respect to their r esear ch pro j-
ect, their career development, and for their educational advancement.
As de scrib ed above, although career devel opment oppor tunit ies are
important for graduate student applicants, they are essential for post-
doctoral trainee s.
For applicants submitting an F30 or F31application, this section
must also include a subsection entitled “Educational Information.”
For PhD trainees (F31 and F31 diversity), this subsection needs to dis-
cuss the overall structure of the graduate program, including the num-
ber and nature of required courses, the milestones of the program (i.e.,
qualifying exams, preliminary exams, oral exams, committee meetings,
etc.), the timeline of when these milestones occur, and any information
detailing teaching that may be required of the applicant. It is also nec-
essary to discuss the average time for students to complete their degree
within each individual program and how the program will monitor the
applicant’s progress. Finally, describe the status of the applicant within
this program. “The applicant is presently in the third year of the train-
ing program and as such has successfully passed her qualifying exami-
nation. She is on track to take her institutional preliminary exam in
approximately 6 months time, at which point she will officially be a
PhD candidate in the graduate program in Genetics.”
For MD/PhD trainees (F30), this subsection needs to discuss the
structure of the MD/PhD program at the institute. This information
will include the years spent in clinical education, the manner in which
the student will select a lab in which to perform their PhD research,
93Last but Not Least: Institutional Environment, Training Potential, and Other Scored Items
the educational requirements during their PhD training (which may be
very similar as described above for the F31 applications), and the sub-
sequent timing and manner in which they transition back to the final
years of medical education. As with many other sections in the grant
application, it is important that MD/PhD trainees include explicit
statements about the clinical aspects of their training environment.
These aspects may include any clinical tutorials that they will take dur-
ing the research years, any aspects of their research that will include
clinical or translational content, the opportunities they will have to
attend grand rounds, tumor boards, or any other clinical seminar or
symposium, and most importantly, any activities or classes that are
required by the MD/PhD program to assist them in transitioning back
into the clinical years of their training once their research is completed.
Oftentimes institutes will already have a “boiler plate template”
that may be used to assist in the writing of the Institutional
Environment and Commitment to Training section. If one does not
exist, it would beneficial for the program to develop such a document.
Further, one of the most effective ways of constructing this section is
to have the director of the graduate program, director of the MD/PhD
program, the departmental graduate coordinator, or the department
chair use the template provided by the institute (if one exists) or write
this section for the applicant, including all of the information described
above. Most importantly, even though a template may be used, this
information must be specialized for each individual trainee! Obviously
certain aspects of the program are the same for all trainees (i.e., clas-
ses, exams, milestones, etc.). However, the description of the research
program and the trainee’s progress through the program must be spe-
cific for that individual. Finally, the individual that provided this infor-
mation must include their name, title, and role within the training
program at the end of the document.
6.2 TRAINING POTENTIAL
Just as the Facilities and Resources, Equipment, and Institutional
Environment and Commitment to Training is one of the most objective
of criteria, the Training Potential is probably one of the most subjective
of criteria during the review process. The Training Potential evaluates
just that, the potential for the applicant to receive an exceptional train-
ing given the information provided in the entire grant application. In
94 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
order to determine this potential, the reviewers consider all aspects of
the training, including the applicant’s qualities, the sponsor’s qualifica-
tions, the sponsor’s training plan, the institutional and intellectual envi-
ronment, the technical aspects of the science, etc. The reason this
section is the most subjective of the five major criteria is because of the
natural bias and differences in opinion that exist in human nature and
affect the review process (discussed in Chapter 2). As such, reviewers
do not always view factors that contribute to the overall training
potential with equal importance. Therefore, by some reviewers the
Training Potential (along with the Institutional Environment) could be
considered to carry a lower “weight” or influence on the overall
strength of the application than the Applicant, Sponsor, and Research
Training Plan. Alternatively, some reviewers may actually use the
Training Potential to guide their decisions for the Overall Impact
score.
Following are several examples of how the evaluation of the train-
ing potential of an application can differ depending on the personal
biases and opinions of individual reviewers. Consider a situation where
you have an exceptional predoctoral applicant (F31 grant); 4.0 under-
graduate GPA, they published a first author paper from their under-
graduate research, stellar letters of recommendation, mature career
goals, and clear understanding of how the present training will help
them achieve these goals. The Research Training Plan is top-notch,
exceptionally written, highly significant, with well laid out experiments.
However, despite the fact that the sponsor has been in his position for
9 years, he has only trained four students. Even though all four of the
students have progressed to exceptional careers, this is considered by
some reviewers to be a low to moderate training experience for the
sponsor and will require the presence of a cosponsor. Therefore,
despite the exceptional nature of the applicant and project, the percep-
tion of a “low training history” (or lack of sheer numbers of trainees)
for the sponsor will negatively influence the training potential of the
applicant for some reviewers.
Now, take as an example the situation where you have a postdoc-
toral applicant whose undergraduate grades weren’t the greatest but
improved throughout graduate school. Because of a difficult graduate
project, the applicant took a little under 7 years to complete their
degree and only published one first author paper. Despite letters of
95Last but Not Least: Institutional Environment, Training Potential, and Other Scored Items
recommendation that attested to the fact that the graduate project was
extremely difficult, thereby explaining the publication record, the lon-
ger graduate career and low publication record is seen as a deficit. The
sponsor is a leader in the field, has trained over 20 postdoctoral fel-
lows, and has a publication list of over 100 papers in highly respected
journals. The Research Training Plan is considered to be one of the
best seen in that study section. In this situation, some reviewers may
consider the academic record of the applicant (regardless of the dem-
onstrated improvement) and the numbers of publications (suggesting a
“poor” productivity despite solid explanations to the contrary) to be
highly important for determining how successful the applicant will be
and how much they will be able to profit from the overall training
plan.
Finally, you may have an applicant that is extremely strong, a spon-
sor who is a leader in their field and has trained large numbers of stu-
dents to highly productive careers. However, the Research Training
Plan is poorly written, lacks focus, has specific aims that are interde-
pendent, and rely on experimental models that are in development and
have not be proven to be feasible. Some reviewers may state that
because of the strength of the sponsor and the applicant, the quality of
the science may not be as critical to the overall training. However,
many reviewers will look at the Research Training Plan and conclude
that the quality of the scientific writing indicates that poor mentoring
was involved in putting together the project and therefore, the training
potential of this application is diminished.
These previous examples may be considered by some to be extreme;
however, they are real life examples from study sections and have con-
tributed to significantly lowered scores for the Training Potential. Of
course more subtle concerns can come into play when a reviewer is
considering the training potential of a grant. A training program at an
institute may not include professional development and/or journal
clubs causing the Institutional Environment to affect the Training
Potential. The sponsor’s training plan, even though it provides details,
may not provide enough details or may be perceived to be “copied and
pasted” from another grant, therefore not considered to be personal
enough. Alternatively, the training plan may focus entirely on the tech-
nical aspects of the training and may not discuss other aspects of pro-
fessional development. Any of these situations may result in lowering
96 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
the score of the Training Potential. Of course, in all of these examples,
the mindset of the reviewers as they are reading your grant will abso-
lutely contribute to how strongly they feel each of these issues affect the
training potential (discussed in Chapter 2). Alternatively, discussions in
study section may bring to light issues that the reviewer may not have
considered, also resulting in a lowered score.
6.3 OVERALL IMPACT SCORE
After evaluating each of the individual criteria described above and
providing a score for each of these sections, the reviewers provide an
Overall Impact Score. Like the determination of the score for the
Training Potential, the Overall Impact Score takes into consideration
all of the sections of the entire grant package: Applicant, Sponsor,
Research Training Plan, Training Potential, and Institutional
Environment. It must be noted that the overall impact score is NOT
the average of the scores of the individual components. Instead, it is a
score that each individual reviewer feels represents the true impact that
the application will have on the overall training of the individual.
Along these lines, many applicants are confused, and possibly even
angry, when they receive their Summary Statement and find that the
Overall Impact Score is actually lower than each of the individual
scores. However, it is important to remember that ALL of the sections
are taken into consideration and examined for how they will contribute
to the overall training of the individual.
For example, consider t he case where the appl icant is excelle nt
and rece ived a score of a 3; the sponsor is solid but wrote a moderate
training plan an d rece ive s a score of a 3; the Researc h Tra ining Pl an
was average and had several weaknesses and also received a 3; the
Institution is solid with no weaknesses and received a 1; and the
Training Potential was considered to be a 3. However, the Overall
Impact score was given a 4. Remember the scoring rubric pres ented
in Chapter 2. While each of the individual components, by strict
adherence to the rubric, are c onsid ered “very strong with some minor
weaknesses” (score of a 3), when the application is considered as a
whole, each section that individually contained some minor weak-
nesses translates into an overall application with numerous minor
weaknesses. Again using strict adherence to the rubric, “strong with
numerous minor weaknesses” equates to a score of a 4. As with the
97Last but Not Least: Institutional Environment, Training Potential, and Other Scored Items
Training Pot ential and as discusse d in Ch apter 2, the biases of the
reviewers, the mindset of the reviewer while they are evaluating
the app licatio n, and opinion s of how strongly a reviewer feels differ-
ent c riteria will affect an overall impact come into play and cannot
be predicted. Furthe r, discu ssi ons in the st udy sectio n (sho uld the
grant be discussed) will also alter the initial impact score provided by
somereviewerstoresultinthefinalimpactscorepresentedtothe
applicant.
6.4 OTHER SCORED ITEMS
In addition to the five major criteria that receive individual scores,
there are other items within the grant application that are considered
scored items but do not individually receive a score. That is, these are
items that may be considered when determining a score for the
Research Training Plan, the Training Potential, or the Overall Impact
score but do not carry as much weight as the five major criteria. These
additional scored items include Vertebrate Animals and the Resource
Sharing Plan.
6.4.1 Vertebrate Animals
If the Research Training Plan proposes the use of an animal model,
then it is a requirement that your application includes a section that
justifies the species being used, the numbers of animals required, veteri-
nary care that will be provided, the procedures that will be used to
ensure the minimization of pain and discomfort, the criterion to be
used to implement euthanasia, and the method by which euthanasia
will be performed. As with every other part of the grant application,
this section requires the inclusion of details:
• Proposed use of animals: Provide detailed information describing
how the animals will be used for the studies described in the
Research Training Plan, including the species, strain, age, sex, and
numbers of animals to be used in the proposed work. For example,
if your Research Training Plan proposes the use of primary cells
derived from neonate mice, you must provide a description of the
strain of mice that will be used, the breeding process you will use to
obtain the neonate mice, how many neonate pups are needed, the
days post birth at which the mouse pups will be euthanized, how the
pups will be euthanized to harvest the tissue, and the surgical
98 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
procedures that you will use to obtain the tissue from which you
will derive the primary cells. Further, if your studies require the use
of only one sex (male vs. female), you must provide a rationale for
why this selection is necessary.
• Justification of the use of animal, species, and number of animals:
Provide detailed information describing why the proposed use of
animal is required and a justification for the numbers of animals
to be used in the study. S ince animal work is costly and in many
cases time-consuming, the r eviewers, and the NIH, want to see a
solid rationale for the use of animals and that similar studies
could not be more effectively performed in a cellular model. Solid
statements of rationale may be “The use of animal models is
essential for understanding the complex proces s of differentiation.
There is presently no in vitro s ystem that can adequately model
the disease stat e in a physiological environment. Further, this ani-
mal model has been chosen because the disease state induced in
this model close ly parallels the disease state observed in humans
and therefore will provide highly relevant translational informa-
tion.” Alternatively, to continue the example used above,
“Although alternatives to primary myoblasts exist in the form of
cell lines, these lines are partially differentiated and have been
manipulated and cultured to achieve immortality, resulting in the
accumulation of genetic mutations that may alter the mo lecular
characteristics of the transcription factor under investigation. ” In
essence, you must demonstrate why there is no other alternative
to the animal model system you propose to use. In addition, you
must provide detaile d information on the numbers o f animals to
be used within your study. This includes th e number of animals
required for the determination of statistical significance within
sets, the total number of sets to be used (controls, mutants, vari-
ous conditions, etc.), with a total animal count to comprise the
entire study.
• Veterinary care: Provide information on the number of veterinarians
on site, their availability in the case of an emergency, how fre-
quently the animals will be inspected by either the veterinarian or a
veterinary technician, and who these inspectors will report to should
they observe any abnormalities (i.e., the attending veterinarian and/
or the principal investigator). This section must also include a
description of how the animals will be housed (e.g., “All mice are
99Last but Not Least: Institutional Environment, Training Potential, and Other Scored Items
housed in top filter ventilated cages on paper bedding with nestlets
and breeding huts when necessary”).
• Procedures for limiting discomfort, pain, and distress: If your pro-
posed use of animals involves surgical procedures or procedures in
which the animals will be restrained, you must provide a description
of how the animals will be treated in order to minimize pain or dis-
comfort. This includes describing the type of anesthetic that will be
used for the surgery (i.e., general or local anesthesia, the amounts of
anesthesia, and a rationale for why the type of anesthesia is being
used) and how postsurgical pain will be managed and monitored. If
animal restraint is required, a rationale for why restraint is unavoid-
able and a description of the method of restraint must be included.
If you believe that the methods to be used will not generate pain or
discomfort, this fact must be stated with a solid rationale for why
this belief is the case.
• Method of euthanasia: If your studies involve a proce ss that wil l
generate a t erminal condition (e.g., tumor f ormation), the endpoint
of study itself involves extreme morbidity (e.g., extreme weight
loss, weight gain, or musc le wasting), or each time point requires
the sacrificing of the animal in order to harvest internal organs for
analysis, then in for mation de scri bing the proce ss es of euthan asia is
required. This i nformation must describe the drug and/or anes-
thetic that will be used, the amount of the drug, and whether the
indicated level of drug is a lethal dose. Further, if extreme morbid-
ity is expected, you must des cribe the p arameters that will be used
to determine that the animal must be euthanized to prevent further
pain and suffering. These parameters may include, but are not lim-
ited to extreme weight loss (indicated by loss of a certain percent-
age of total body weight), unresponsiveness to immedi ate
interventions, or extreme lethargy or inability to consume food or
liquids. Finally, your description must explicitly state that the cho-
sen method of euthanasia is consiste nt with the re commendations
of t he Panel on Euthanasia of the American Veterinary Medica l
Association.
6.4.2 Resource Sharing Plan
Many times the work performed in the Research Training Plan will
generate unique resources, which can be in the form of reagents, such
as antibodies or DNA constructs, model organisms, or large data sets,
such as whole genome sequencing or genome-wide association studies.
100 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
The sharing of these unique resources is integral for the enhancement
of research and facilitates advancing work in the field or science in
general. When these unique resources have been developed through the
use of NIH funds, it is a requirement that these resources be made
readily available to the scientific community. The Resource Sharing
Plan is the description of how you, the investigator, will make these
reagents available to the scientific public. These methods of sharing
may include placing the large data sets on a public access database for
which a web address will be provided, sending of novel reagents when
requested by an individual, or providing animals for breeding upon
request.
101Last but Not Least: Institutional Environment, Training Potential, and Other Scored Items

CHAPTER
7
7
Details, Details, Details—Nonscored Items,
Formatting, and the Cover Letter
Several components of the application package are considered “non-
scored” items. These sections contain information that will be used by
the National Institutes of Health (NIH) for public information
purposes should your grant be funded (Project Summary and Project
Narrative) or to ensure that your training will be in compliance with
NIH guidelines (Responsible Conduct of Research). These items
neither contribute to the individual scores nor will they influence the
overall impact score of your grant application. However, it is still
essential that the utmost care be used in writing each of these sections.
7.1 PROJECT SUMMARY/ABST RACT (30 LINES)
The project summary is the abstract for the Research Training Plan
and as such serves as a succinct description of the proposed work.
Should the application be funded, the NIH will publish the Project
Summary online on the NIH Reporter database (
http://projectreporter.
nih.gov/reporter.cfm
). Therefore, the Project Summary must serve as a
stand-alone document. Further, it must be written so that anyone sci-
entifically or technically literate will be able to understand it. Because
it serves as an overview of the project, the Project Summary must
include background information, overall objectives, a statement of the
hypothesis to be tested, the evidence or data that allowed the develop-
ment of the hypothesis or supports the feasibility of the project, the
Aims to be tested and the methods used to address these aims, and a
reference to the health relatedness of the work.
Because the Project Summary succinctly summarizes the research
project, it is often best to write this section after the Research Training
Plan has been completed. Therefore, it is easy to take sentences written
for the research plan and modify them slightly for the Project
A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant. DOI: https://doi.org/10.1016/B978-0-12-815336-9.00007-7
© 2018 Elsevier Inc. All rights reserved.
Summary. A general rubric that assists in constructing the Project
Summary can be as follows:
• Begin the paragraph with two to three sentences describing the
background of the project. You want to provide enough informa-
tion so that the reader can understand the overall field and have
perspective for how your project fits into this field. Along these
lines, it is often good to lead off with a catchy sentence that high-
lights the severity of a disease or puts a scientific question in per-
spective as it relates to human health: “Chronic alcohol
consumption alters metabolic regulation, which can lead to muscle
wasting, one of the most recognized factors affecting morbidity in
these individuals.” To facilitate writing these opening sentences, it is
helpful to condense and paraphrase the opening paragraph of your
Specific Aims page.
• Once you have established the background, explicitly state the gap
in knowledge and why this gap is a problem to advancing treat-
ments: “However, the underlying molecular mechanisms that con-
tribute to the development of chronic alcohol associated muscle
wasting are not fully understood. Without this knowledge the ability
to develop novel therapies to reduce morbidity associated with this
condition will be greatly inhibited.”
• Once you have stated the gap in knowledge, provide additional
information from published literature evidence or preliminary data
that allowed you to develop your hypothesis. This needs to be done
in one to three sentences. As with the opening sentences, it is recom-
mended to condense and paraphrase the literature evidence and pre-
liminary data as stated on your Specific Aims page.
• Having provided the required information, state your hypothesis:
“Therefore, we hypothesize that...” being very specific about what
your hypothesis is. For example, if you are examining the role of a
specific molecule in a signaling pathway and how that signaling is
altered in a disease state: “Therefore, we hypothesize that the pres-
ence of chemokines in the proinflammatory environment associated
with chronic alcohol consumption alters signaling ultimately dis-
rupting the expression of genes associated with muscle wasting. ” It
is recommended to use the exact wording for the statement of the
hypothesis in the Project Summary that you used on the Specific
Aims page.
104 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
• Then, state your specific aims: “We will address this hypothesis
through the following Specific Aims...” making sure that the spe-
cific aims you describe here are written identically to those written
in the Research Training Plan.”
• Follow-up the statement of the aims with a very brief (one to two
sentences) description of the experimental model system and how
this model system will be used to address the aims. “We will utilize
muscle satellite cells derived from our chronic binge alcohol animal
model as our experimental model. We will treat these cells with
proinflammatory cytokines and monitor molecules of the signaling
pathway along with the transcriptional activity of the key transcrip-
tion factor and the expression of genes essential for protein
degradation.”
• Wrap-up the paragraph by summarizing how you expect the suc-
cessful completion of this project to advance the field and how this
advancement will contribute to the health relevance of the disease
and/or health-related issue you are studying. “The information
obtained from this project will provide a solid groundwork to estab-
lish a model by which proinflammatory molecules contribute to
muscle wasting. This model will thereby enable more detailed stud-
ies aimed at determining how these molecular mechanisms can be
exploited to develop novel therapies to alleviate the morbidity asso-
ciated with this disease.”
After providing a statement of the health relevance of the project,
applicants sometimes include a statement that describes how this proj-
ect, or more generally the overall training plan, will provide a frame-
work for an excellent training environment. This statement is not
required and in fact the inclusion or failure to include such a statement
does not detract from the overall summary.
7.2 PROJECT NARRATIVE (23 SENTENCES)
The Project Narrative is a statement that will be used for public dis-
semination and describes how your project is relevant to public health.
This section is very short and consists of only two to three sentences.
Because this element is used for public release, it must be written in a
language that can be understood by the general, lay audience.
Therefore, do not use jargon or highly technical terms but instead use
broad language that focuses on the general impact that the project will
105Details, Details, Details—Nonscored Items, Formatting, and the Cover Letter
have to a particular disease or health-related issues. For example:
“Rhabdomyosarcoma is an aggressive childhood solid muscle tumor
with a poor prognosis that is characterized by a specific oncogenic pro-
tein. Understanding how this oncogenic protein changes global
miRNA and gene expression thereby altering transcriptional regula-
tory networks to affect normal muscle development will assist in identi-
fying new targets that could be exploited for the rational design of
drugs for the treatment of this tumor.”
7.3 RESPONSIBLE CONDUCT OF RESEARCH (1 PAGE)
In 1989, the NIH established a policy concerning the teaching of
responsible conduct of research. This policy requires that any training
grant application must include a description of how the applicant
will be instructed in the ethical issues related to basic research (
http://
grants.nih.gov/grants/guide/notice-files/NOT-OD-10-019.html
). The
responsible conduct of research encompasses many aspects of ethical
behavior and is not limited to research misconduct, which refers to the
fabrication, falsification, or plagiarism of work in proposals or
published materials. Therefore, responsible conduct of research is the
practice of scientific investigation with integrity and involves the
awareness and application of established professional norms and ethi-
cal principles in the performance of ALL activities related to scientific
research. Research training grants that lack a description of these types
of instruction may be returned without review.
The NIH has established explicit information that is required for an
acceptable description on the instruction in the Responsible Conduct
of Research:
• Class format: Include a description of the format the instruction
takes. Will the instruction include didactic lecture, group discus-
sions, or both? What will be the source material for the lecture and/
or discussion (i.e., text book, essays, handouts)? How will perfor-
mance in the class be determined (i.e., exam, take home essays, in
class participation, etc.)?
• Subject matter: Include an explicit description of the topics to be
covered in the instruction. This is most easily addressed by including
a list of all of the lecture and/or class topics included in the courses
the applicant will take, best found in the syllabus for the class. The
106 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
NIH does not have specific curricular requirements for the instruc-
tion. However, several topics are recommended that constitute satis-
factory training. These topics include conflict of interest, use of
human subjects or vertebrate animals, mentor/mentee responsibili-
ties and relationships, collaborative research, peer review, laboratory
tools and management, intellectual sharing and ownership, research
misconduct, authorship on publications, and the scientist as a
responsible member of society.
• Faculty participation: Include a description of how faculty will par-
ticipate in the instruction. Will a single faculty member lead the
class? Will several faculty members contribute in a team-taught for-
mat each lecturing on a topic given their area of expertise?
• Duration of instruction: Provide a description of how long the class
and/or instruction will last (i.e., 6 weeks, one semester, etc.)
• Frequency of instruction: Provide a description of how frequently the
class will meet. This information can be combined with the duration
as in the following example: “The course met/will meet for 1 h each
week for the duration of the fall semester.”
In addition to the formal instruction just described, it is also
acceptable to include a statement that is tailored to the individual
applicant and includes one-on-one mentoring from the sponsor as it
relates to general scientific integrity or ethical issues associated with
the specific research activities. While these latter discussions are
acceptable as supplementary information, one-on-one instruction with
the mentor will not be considered a substitute for formal classroom
instruction. Further, as the Responsible Conduct of Research is not a
scored item, a poorly written section will not prevent the funding of an
otherwise exceptional grant. The applicant will simply have to rectify
the problematic issues to be acceptable to the NIH before the grant is
officially awarded.
7.4 FORMATTING
The NIH has very specific guidelines regarding formatting and these
guidelines apply to all sections of the grant application. These guide-
lines are in place to make sure consistency exists in the presentation of
grant applications, thereby facilitating the ease with which the applica-
tions can be read. These guidelines also prevent the utilization of
smaller fonts that would allow increased amounts of information to be
107Details, Details, Details—Nonscored Items, Formatting, and the Cover Letter
included within the application, thereby providing equity between all
grant applications. These formats are to be strictly adhered to and fail-
ure to do so may result in the grant being administratively rejected
without review. These formatting specifications are as follows:
• Font: Arial, Helvetica, Palatino Linotype, or Georgia typeface, 11
point or larger, with a black font color.
• Type density: May be no more than six lines per inch, which equates
roughly to a single spaced distance between lines of type.
• Paper size and margins: 8.5v 3 11v paper size is required with a
minimum of 0.5-inch margins on top, bottom, left, and right.
• Page formatting: Use only a “single column” format (instead of
double column format) and do not include headers or footers.
• Page numbering: It is not required. Page numbers will be system-
generated in the complete application upon submission, with pages
numbered sequentially for all parts of the grant.
• Figures, Graphs, and Tables: Figure legends, table descriptors, and
text within graphs and charts may be smaller than the 11 point
required for the body of the text. However, you must use the same
font in the legends and descriptors as you do in the body of the text.
Further, the font size cannot be any smaller than is legible when
viewed at normal size or printed out onto 8.5v 3 11v paper.
• Page limits: The page limits for each section, as dictated by the
application guide are strictly adhered to. Failure to stay within these
page limits will result in an administrative rejection without review.
In addition to these official formatting guidelines, it is important to
keep in mind the overall appearance of the application. This fact was
discussed previously in the presentation of figures in the Preliminary
Data section of the Research Training Plan (see Chapter 5). However,
this attention to overall appearance also comes into play in the actual
text of the application, in particular in the visual density of the text
and the complexity of writing. When constructing your application it
is essential that you use shorter, more declarative statements that flow
from one sentence and paragraph to the next and that you create
“white space” in your text, thereby providing visual rest for the
reader’s eye.
To illustrate this point it’s often good to keep in mind that you
want to write like Hemingway, not Faulkner. Ernest Hemingway and
William Faulkner, two giants of American literature, both Nobel
108 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
laureates and Pulitzer Prize winners .. . two VERY different writing
styles. William Faulkner revolutionized the way the English language
was used in order to capture the workings of the human mind, many
times describing a tortured mind in its descent into insanity using
stream of consciousness writing. Hemingway examined how human
kind react to harsh situations, how they exhibit “grace under pressure.”
Although both men captured the deep emotional, political, and social
complexities of their time and world, they used very different writing
styles in order to do this. Faulkner wrote long, complex, seemingly
wandering sentences that covered several pages. These sentences were
punctuated with parenthetical phrases, italics, and even a lack of punc-
tuation to represent confusion and seemingly chaotic wanderings of
the human mind. In contrast, Hemingway created and perfected what
is now known as “shotgun prose.” He used short declarative sentences
that are straight to the point. However, these short declarative sen-
tences flow to create a work that suggest deeper meanings than what is
physically stated on the page. Hemingway said more through what
was left unsaid, through the underlying meaning of the words on the
page, than many authors are capable of doing in several pages of text.
Visually these writing styles are very different, too. Faulkner’s writ-
ing appears very dense on the page, with paragraphs that go on for
several pages leaving very little white space, or visual rest for the read-
er’s eye. Hemingway’s writing, on the other hand, has shorter para-
graphs punctuated with dialog, thereby creating large amounts of
white space and visual rest for the eye. It is this latter appearance that
you need to strive for in the writing of your application. This can be
done relatively easily:
• Provide a line of space between paragraphs.
• Write shorter paragraphs that contain one defined concept or idea
and if you find that a paragraph consumes a majority of the page,
find a way to break it up into smaller, bite sized thoughts and
paragraphs.
• Use shorter, declarative sentences that flow one into the other, going
for the “shot gun prose” style of Hemingway over the long, convo-
luted style of Faulkner. As with the paragraphs, if you find a sen-
tence continuing on for many lines of text, find a way to
grammatically separate it into individual sentences.
109Details, Details, Details—Nonscored Items, Formatting, and the Cover Letter

Although the concept of visual “white space” may not seem impor-
tant, examine the difference in appearance between a Research
Training Plan that does not follow this suggestion (
Fig. 7.1) and one
that does (
Fig. 7.2). Remember, the person reviewing your grant may
be approaching this task under adverse conditions. Opening an appli-
cation and being faced with a dense page of text with little to no
“breathing room” for the eye may seem very daunting to that reviewer,
thereby giving them an unintentional negative first impression.
Figure 7.1 Illustration of minimal “white space” in a grant application.
110 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant

In contrast, the visible white space on the page immediately lets them
feel that they can read and digest your writing in small, discrete
sections, which can be a relief for a tired reviewer.
One last thing to consider when writing your application—be very
sparing with the use of acronyms! While acronyms can be good when
writing a document with very limited space constraints, the use of too
many acronyms can become overly confusing, ultimately annoying
what may be a very tired reviewer. Remember, although the acronyms
Figure 7.2 Illustration of the inclusion of “ white space” in a grant application.
111Details, Details, Details—Nonscored Items, Formatting, and the Cover Letter
that you use may be familiar to you and to people in your field, these
same acronyms will not necessarily be familiar to the person reading
your application, who most likely is not in your field. When you use
acronyms you are asking the reader to remember their meaning as
they read through the application. The use of too many acronyms
therefore can become burdensome on the reader, many times requiring
them to search back through the document to remind themselves of
their original definition. Therefore, a good rule of thumb is if you
know that a term will be used continually throughout the document
[e.g., fluorescence in situ hybridization (FISH)] then use the acronym,
spelling it out the first time it is encountered with the acronym immedi-
ately following it in parentheses. If you know that the term will be
used minimally (e.g., only once or twice), then it is better to simply
spell out the term.
7.5 COVER LETTER
Part of ensuring that the grant application receives a fair review is
making sure that it is sent to the most appropriate study section. As
described in Chapter 2, a majority of the study sections associated with
the Ruth L. Kirschstein training grants are interdisciplinary and there-
fore focus on a particular scientific topic and/or discipline. While some
of these disciplines may overlap, they each have a very distinct focus.
As such, the reviewers on these panels have very different ways of
thinking about science. For example, a grant may contain a research
training plan that discusses the role of a transcription factor in
regulating gene expression during muscle development. The research
examines a molecular mechanism important in muscle development
and proposes experiments that are molecular biological and bio-
chemical in nature. Technically, this grant should be directed to the
study section on Genes, Genomes, and Genetics, which review grants
that relate to the regulation of gene expression. However, because the
research examines transcriptional regulation in the context of muscle
development, it could also be considered for the study section on Cell
Biology and Development.
On the surface either section would seem appropriate. However,
consider the research focus of the members of these study sections.
Many reviewers on the Genes, Genomes, and Genetics study section
112 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
perform molecular biological, biochemical, and genetic work and can
therefore appreciate the line of proposed experiments. In contrast,
many of the members on the Cell Biology and Development study
section perform more developmental biology work and would expect
and/or want to see a line of experiments that utilize more developmen-
tal biology. Although it seems as if assignment to the latter section
would be okay, in actuality, the grant may not receive an appropriate
review simply due to differences in scientific experience.
The assignment of grant applications to study sections occurs in the
Division of Receipt and Referral at the Center for Scientific Review. It
is within this division that the content of the scientific plan is examined
and a decision as to the most appropriate study section is made. Since
people who are not intimately familiar with the work are making deci-
sions on where the application is assigned, the applicant can facilitate
this process, and influence the assignment of the application, by
requesting a study section and providing explicit logic within the cover
letter as to why this section is the most appropriate. Along these lines,
the NIH has developed a recommended format when including assign-
ment requests in the cover letter, which is as follows:
“Please accept for consideration the Ruth L. Kirschstein National Research
Service Awards for Individual Predoctoral Fellows (Parent F31) grant applica-
tion entitled [insert title] in response to announcement [insert the NIH pro-
gram announcement number].
Please assign this application to the following:
Institute/Center:
National Cancer Institute—NCI
Scientific Review Group:
IMST—Interdisciplinary Molecular Sciences and Training
Fellowship: Oncological Sciences [F09]
The reasons for this request are....”
Follow this statement by one short paragraph outlining your ratio-
nale for this request. When describing the rationale, it is often advis-
able to quote directly from the description of the topics covered by
that study section. “The stated focus of the Oncological Sciences
IMST study section is to ‘review applications involving the pathology
of the malignant cell... with an emphasis on mechanisms... and
molecular events in gene regulation.’ Among the stated specific areas
covered are ‘gene regulation including... transcription... relevant to
113Details, Details, Details—Nonscored Items, Formatting, and the Cover Letter
oncogenesis.’” This rationalization is followed by a second short para-
graph in which you briefly describe the research in the proposal and
state exactly how this research fits within the scope of the topics con-
sidered by the desired study section. Finally, a cover letter for the
Ruth L. Kirschstein training grants also requires the inclusion of the
name, degree, position, and affiliation of the individuals who have
agreed to submit reference letters.
114 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant

CHAPTER
8
8
Now What?—Resubmission
Once you’ve submitted your grant, then the waiting begins. Grants
submitted for the April 8 deadline will be assigned to a study section
approximately a month later. Once assigned, these study sections will
meet anywhere between late June and mid-July. The study section will
last 12 days, depending on the number of grants under consideration,
after which the Scientific Review Officer must compile the scores for
notification. Once compiled, these scores will be posted on individual
eRA Commons sites, a process which may take another week or lon-
ger. However, at this point the applicant will only know whether their
grant was “not discussed,” whether they received a score and if so,
what that score is. Further, unless the score is better than 20, the appli-
cant will also not know with absolute certainty whether the grant will
be funded. As discussed in Chapter 2, each individual institute deter-
mines the funding levels and what scores will be funded. In addition,
the applicant will not know why they received the score they did as the
reviews, in the form of a Summary Statement, will not be released for
about another month. Therefore, it may be upwards of 3.54 months
from the time a grant application is submitted until receipt of the
critiques.
Before discussing the Summary Statement and how to address com-
ments, a brief discussion of how the final score is presented is needed.
As described up to this point, the reviewers provide single integer num-
bers from 1 to 9 for each of the individual components and for the
overall impact score. These single integer numbers are used in study
section to establish a range for voting. When the final votes are entered
following discussion, each member submits their decision as a single
digit number from 1 to 9. As described in Chapter 2, the entries from
all of the members are then averaged to provide the final score for a
particular grant. When reported to the applicant, the score is multi-
plied by 10. For example, assume there are eight members on a study
A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant. DOI: https://doi.org/10.1016/B978-0-12-815336-9.00008-9
© 2018 Elsevier Inc. All rights reserved.
section. After discussion the scores these members enter are 1, 2, 4, 3, 3,
2, 2, and 3. This range of scores would result in an average score of 2.5,
which would then be reported to the applicant as a score of 25.
8.1 THE SUMMARY STATEMENT
The Summary Statement is the document that summarizes the overall
review of the applicant’s grant. If the application received a score,
which means that the proposal was discussed in the study section, the
statement will begin with a paragraph entitled “Resume and Summary
of Discussion.” This paragraph is written by the Scientific Review
Officer and summarizes the discussion that occurred for this grant dur-
ing the meeting, detailing the score driving factors with respect to
strengths and weakness of the grant application. If the grant did not
receive a score, which means that it was not discussed, there will be no
summary included.
Following the summary of the discussion, the Summary Statement
next presents the critiques of each of the three reviewers who read your
application. These reviews are separated into Reviewer #1, Reviewer
#2, and Reviewer #3 with each section beginning with a listing of the
five different criteria (Fellowship Applicant, Sponsors/Collaborators/
Consultants, Research Training Plan, Training Potential, Institutional
Environment and Commitment to Training) and the individual priority
score (19) that the reviewer assigned to each individual criterion.
This score description is followed by a paragraph entitled “Overall
Impact/Merit” in which the reviewer summarizes their opinion of the
overall quality of the application with statements of what they felt the
strengths of the application were and what weaknesses detracted from
the overall quality of the grant. Next in the Summary Statement are
individual sections with headings for each of the five criteria. Under
these headings are subheadings for “Strengths” and “Weaknesses,”
where the reviewer provides bulleted, detailed, and hopefully construc-
tive comments about what they felt were the strengths and/or weak-
nesses for that particular section in the application. Completing the
Summary Statement will be a list of the members of the study section
with their academic rank and affiliation. Remember, these reviews are
anonymous, which means that although you know who was on the
study section, you will not know which members of the study section
reviewed your application.
116 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
As discussed in Chapter 2, only Reviewers #1 and #2 are required
to provide individual scores for each criterion, provide an overall
impact statement, and fully comment on the strengths and weaknesses
of each of the individual sections. Reviewer #3 needs only to provide
individual scores and write an overall impact statement. In a perfect
world, all of the reviewers would provide solid, rational, detailed, and
constructive comments that will direct the applicant in addressing the
perceived weaknesses of the grant. Further, the comments provided
should also justify and/or coincide with the scores given based on the
NIH scoring rubric. However, the reality is that many times an
applicant will receive reviews for a section in which “no weaknesses
were noted.” The absence of any noted weakness, if strict adherence to
the NIH rubric was followed, should coincide with a score of a 1.
However, many times even though a reviewer noted no weaknesses,
they will still give a score of a 3 for that section. A score of a 3
indicates that minor weaknesses should be present and require a
rationalization for why that score was given.
It is also possible that even though the comments and scores coin-
cide as described in the scoring rubric, the comments are vague or indi-
cate that the reviewer has not carefully read the application. For
example, a grant could be submitted in which the sites of protein modi-
fication have been identified and published. Based on this published
knowledge, the Research Training Plan then focuses on determining
the biological relevance of each of these identified protein modifica-
tions. In the review the application receives a comment, “The proposal
could focus more on the overall importance of the modification rather
than just identifying specific sites of modification.” It is apparent that
the reviewer did not read the application closely or if they did, they
were not careful in writing a proper critique. These types of inconsis-
tencies are frustrating and ultimately not necessarily fair. However,
fair or not, the discussion of human nature and natural biases that
occur during the review process must be kept in mind and ultimately it
is the job of the applicant to address the concerns of these reviews.
8.2 TO RESUBMIT OR NOT TO RESUBMIT
The question to resubmit is always important to ask when receiving
the results and reviews of a grant application. Sometimes the question
117Now What?—Resubmission
is very easy to answer. If the grant received a score of 20 or better it
will most likely be funded and resubmission is not necessary.
Conversely, if a grant is not discussed and the reviewers consistently
gave individual scores of 5 or worse, a resubmission of the grant would
most likely be a futile exercise. However, there are definitely gray areas
where the decision to resubmit may not be as cut and dried as the two
scenarios just given. As with all aspects of submitting a Ruth L.
Kirschstein training grant, the decision to resubmit is a case-by-case
basis and will be different for each application depending on the com-
ments and critiques.
When deciding whether to resubmit, it is important to remember
how the decision to discuss grants is determined (see Chapter 2). If
there are ,810 applications for a particular section (i.e., F30, F31,
F32, etc.), all of the grants will be discussed and as such all of the
grants within this section will receive a score. Therefore, a grant may
receive a score, but that score may be a 60 or worse. In this case, it
would not necessarily be beneficial to resubmit since overall that
application was perceived as being significantly flawed. In contrast, if
there are more than 810 grants (as with the F32 postdoctoral grants
which may have upward of 6070 submissions in a study section), then
only the top 50% of the grants will be discussed with the cutoff point
being determined by how the scores naturally divide. In this situation, it
is highly possible that a grant may not be discussed but in actuality
have received an initial impact score in the low 30s, which technically
may be close to a fundable level as some institutes within the NIH may
fund grants that have scores in the upper 20s. In this latter illustration,
this application would definitely be worth revising for resubmission.
The next question that arises is if the application was not discussed,
in which case the initial impact score cannot be known, how does one
determine if the overall impression of the study section was favorable
and that the grant is worth resubmitting? An examination of the indi-
vidual criterion scores from each reviewer will indicate the overall
impression. Further, chances are that a majority of the reviewers’ com-
ments will be constructive and detailed, providing insight into the
strengths and weaknesses of the grant. Therefore, if a majority of the
individual criterion scores are 4s, 3s, or 2s with solid, positive critiques
and these critiques are easily addressable in a revision, then the overall
118 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
impression was favorable and resubmission should be considered. Even
if a not-discussed grant has poor individual criterion scores and unfa-
vorable critiques, it is possible that a grant application could be rewrit-
ten and revised significantly to improve its score. Ultimately, the
decision to resubmit is up to each individual applicant and sponsor
and needs to be determined based on the general impressions given
through the Summary Statement.
8.3 ADDRESSING THE CRITIQUES
During the review process, a resubmitted grant will be given an A1 sta-
tus (i.e., CA123456-A1), which indicates that the application in front
of the reviewer is a resubmission. As a resubmission, there is one addi-
tional criterion added to the review process, a criterion that does not
receive its own individual score but whose evaluation contributes to
the overall impact score: How well did the resubmitted application
address the previous reviewers’ comments? When a reviewer is evaluat-
ing a resubmitted grant, they will have access to the Summary
Statement from the previous submission, which will allow them to
know the previous reviewers opinions of the strengths and weaknesses
of the previous submission. Whether these prior critiques were
addressed or the quality with which these previous critiques were
addressed will then be considered in the resubmitted form.
At this point, an important note must be made about the reviewing
of resubmitted grants. The makeup of study sections for the Ruth L.
Kirschstein grants is ad hoc, which means that the composition is not
constant and usually changes from study section to study section.
There will be several members who routinely serve on a particular
study section; however, none of the members is assigned to a single
section on a permanent basis. Therefore, it is more than likely that the
people who reviewed a grant application on the first submission will
NOT review the grant upon resubmission. Further, even among the
reviewers who have served on multiple study sections for the same
topic, it is highly unlikely that they will be assigned a resubmitted
grant on which they served as one of the original reviewers. Finally,
even if a person is assigned a resubmitted grant that they reviewed on
its first submission, they will not necessarily remember having reviewed
it the first time around. Remember, it has been approximately 4 months
or longer since they would have originally seen this grant. If they were
119Now What?—Resubmission
not able to serve on the subsequent study section, upwards of 8 months
may have elapsed. In that time period, they will have reviewed at least
24 (or more) other grants and discussed about 100 grants (or more) in
total. It is a good rule of thumb when rewriting a grant for resubmis-
sion, in particular in writing the Introduction (see below), to assume
that the person reading the resubmission will not have seen the original
submission and if they did, they will not remember having seen it
before. Therefore, the assessment of the resubmission, regardless of
who is reading it, will rely almost solely on the previous critiques and
how well these critiques were addressed.
In the world of sales, there is a saying that the customer is always
right. When an applicant sits down to revise a grant for resubmission a
similar saying could apply: The reviewer is always correct. In essence,
if the reviewer says jump, the applicant should say “how high.” These
sayings are not meant to be taken literally. Of course there are situa-
tions in which a reviewer will provide a critique that makes it evident
that they did not fully understand the point that was being made or
simply did not read the grant carefully enough. However, the saying
above is meant to indicate that if the reviewer wrote a critique, regard-
less of how much you agree or disagree with it, this critique must be
taken into consideration when revising the grant and must be
addressed in the revision. If not, neglecting to do so will be noted
when the resubmission goes to study section and this neglect may nega-
tively influence the overall score.
When revising a proposal, it is very important that the applicant
tries their best to put their pride away and approach the process with
the perspective that the reviewer’s comments are valid and are intended
to improve the grant. Sometimes this mind set needs to develop over a
short period of time after the initial shock, anger, and frustration of
the score and the perceived injustice of the comments wears off.
However, even in the case where a comment is made by a reviewer
that suggests careless reading, the applicant would do well to take a
step back, dissociate themselves from what was written in the first sub-
mission, and make the assumption that they did not present their point
as clearly or logically as they thought they did. In essence, the reviewer
was correct, maybe not in what they said or how they said it, but in
the sense that they highlighted something that needs to be improved
for clarity.
120 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
Some of the critiques are straightforward to address. If a sponsor
has a moderate but not stellar training history and a reviewer com-
ments on this fact, then in the resubmission, a cosponsor needs to be
included using all of the qualifications discussed in Chapter 4. If the
sponsor does not have funding to cover the entirety of the training
period, they must include solid and detailed descriptions for exactly
how the applicant will be supported financially. If there are experi-
mental issues that were called into question, the Research Training
Plan must be modified according to the critiques. If the applicant
proposes the use of a cutting-edge technique but provides no evidence
that the expertise is present in the lab or at the institute to perform the
experiments or to evaluate the results, then a collaborator must be
included in the resubmission along with a letter of support. If it is felt
that there is not enough preliminary data to support the feasibility of
the project, this preliminary data must be included in the resubmission.
It is through these critiques that the reviewers are telling the applicant
exactly what is needed to improve the application.
There are other situations that may not necessarily be as easy to
address. If a reviewer comments about the poor academic performance
of the applicant, there is nothing that can be done to change this fact
(see Chapter 3). The past is the past. However, the applicant can
address this issue by providing a statement in the revised Personal
Statement about why they had a poor academic history and how this
history will not or does not affect their present and future capabilities
as an independent scientist. The applicant could also have a referee
from his previous institute comment that the previous academic perfor-
mance is not reflective of their current ability. In another case, an
applicant has chosen to remain at their graduate institute to perform
their postdoctoral work, a situation that is not looked upon favorably
by reviewers. Therefore, in the revision include a statement in the
Personal Statement of the Biosketch and the Selection of Sponsor and
Institute as to why this choice was made and provide evidence that the
present training is different from past training experiences (e.g., differ-
ent department, different model system, etc.). Regardless of the
critique, the issue that the critique raised must be addressed and it
must be addressed in a logical, clear, and very prominent manner.
121Now What?—Resubmission
Finally, sometimes the reviewer is just wrong or sometimes there
are simply just differences in opinion or differences in the interpreta-
tion of data or experimental design between the applicant and the
reviewer. Although the first instinct is to merely brush aside the cri-
tique as being unfounded or wrong and ignore it, the comment must
be addressed. However, because these are differences in opinion or
cases where the reviewer simply did not take note of something that
was already in the grant, these issues need not be addressed directly in
the body of the application. Instead, they should be professionally and
respectfully addressed in the section called “Introduction to the
Application” (see below). Ultimately, if the reviewer has commented
on any aspect of the application, that comment must be considered
and addressed in some form or another within the revised application.
8.4 INTRODUCTION TO THE APPLICATION (1 PAGE)
A resubmitted grant is required to have an additional section entitled
“Introduction to the Application.” It is within this section that the
applicant specifically details how they have addressed the critiques of
the reviewers from the original submission. This section is extremely
important because, as described above, it is very possible that the
reviewers reading the resubmitted application most likely were not the
people who read the original submission and if they were, they will not
necessarily remember the details. Therefore, it is critical that the
Introduction be carefully written to concisely point out the deficiencies
noted in the first submission, explicitly point out how these deficiencies
were addressed, and direct the reader to the place in the revised appli-
cation where the modifications were incorporated.
When writing the Introduction, it is good to start with a sentence
reminding the reviewer that they are, in fact, reading a resubmission
and when the previous submission occurred: “This is a revised version
of the proposal [enter the assigned grant number], which was originally
submitted in August of 20XX.” This information provides the reader
with a time frame between the original submission and the present
resubmission, which allows them to evaluate whether the amount of
productivity seen in the revision is consistent with the time that the
applicant had to produce the work. Follow this statement by briefly
pointing out the strengths of the original submission by using exact
quotes from the previous reviewers: “Several strengths were noted, in
122 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
particular that the original submission was ‘focused and significant,’ as
well as ‘an excellent proposal from an excellent applicant working in
an excellent environment under the sponsorship of an excellent, well-
funded mentor.’”
Once the strengths of the original submission are noted, be sure to
respectfully recognize the value of the critiques of the previous
reviewers: “In addition to these strengths several criticisms were pre-
sented, which we have addressed and subsequently produced what we
feel to be an improved proposal.” At this point provide either a bul-
leted or numbered list of the previous critiques with a concise, yet
detailed description of how these critiques have been addressed. It is a
good idea to explicitly quote the previous reviews, or an abbreviated
form of the previous reviews, to provide evidence of what was said and
include detailed information regarding where in the resubmission the
reader will find these modifications:
“Reviewer #1: The biological significance of the proposed mutations is not
clear beyond the information already known.
Response: We provide solid experimental data demonstrating the biologi-
cal significance of the mutations at each individual site as it relates to the
known biological properties of the cellular model (Table 3.1 and Figures 2
and 4).”
When addressing the critiques there are three general types of
responses that are needed depending on the nature of the critique: (1)
addressing critiques that explicitly change and/or modify the original
proposal; (2) addressing critiques that cannot be changed; and (3)
addressing critiques in which there is an honest difference in opinion
between the applicant and the reviewer. Each of these situations needs
to be handled differently when writing the Introduction. The first situa-
tion, in which there is the inclusion of additional data to support the
point, or the addition of a cosponsor to augment what was considered
a poor training history, are fairly straightforward, as illustrated in the
above example. The second situation is probably the most difficult to
address since this usually involves problems with the history of the
applicant that can no longer be changed. In this case, it is best to
directly recognize the point that was made by the reviewer and then
provide information that will dispel their concerns: “I recognize that
my grades in my undergraduate years were not optimal. However, dur-
ing this time I was dealing with several personal issues that distracted
123Now What?—Resubmission
my focus (see applicant Personal Statement). Once these personal
issues were resolved, and my focus returned, my academic performance
improved, as evidenced by my improved grades in graduate school.”
This statement recognizes that a problem existed, it provides an expla-
nation that any human being would understand, and it demonstrates
that this apparent setback was temporary and does not affect the pres-
ent training period or the future prospects of the applicant.
The third situation, while apparently straightforward to address, is
also the trickiest. In the situation where there are differences in
scientific opinion between the reviewer and the applicant, no direct
modifications are necessarily made to the application. Therefore, the
viewpoint or opinion of the applicant needs to be explicitly stated in
the Introduction where the applicant is arguing their opinion to the
new reviewer. Ultimately, the new reviewer makes the decision on who
they think is correct, a decision that is based on the previous critiques,
the applicant’s rebuttal, and their own interpretation from reading the
application. However, it is still possible to write the response such that
the scientific point is made respectfully and ultimately assuming the
viewpoint that the original logic may not have been written as clearly
as it could have been. “The applicant respectfully disagrees with this
reviewer’s assessment of the data. In the original application we stated
that [explicitly quote the statement from the original submission].
Along these lines, we feel that literature evidence firmly supports the
conclusion that [state the conclusion that you believe to be correct].
However, the interpretation of the results may have resulted from a
lack of clarity in the writing. Therefore, we have revised the writing for
clarity and included additional references to support our conclusion.”
Of course these three scenarios are generalizations. However, a
majority of the critiques will usually fall under these three broad con-
cepts. As with all sections of the application, the type of responses that
are required will be different for each individual application and appli-
cant and may not necessarily be easy to address. Regardless of how
much the applicant agrees with the critiques or feels that the critiques
are fair, it is essential to address the reviewers’ comments head on
rather than ignore any of them. The reviewers of the resubmission
usually read the applications very carefully, especially a revised appli-
cation, and very little will get past them. The neglect of a previous
comment by the applicant will be noted and most times will negatively
124 A Practical Guide to Writing a Ruth L. Kirschstein NRSA Grant
affect the review of the resubmission. In fact the scores for a majority
of resubmitted grants do not improve significantly, or even are poorer
relative to the original submission simply because the applicant did not
feel it was necessary to address all of the critiques.
Another note that must be made is that many times the nature of
the three reviewers’ comments is very similar. For example, all three
may comment on poor grades or lack of publications. Two of the three
may have commented on the quality of a particular experiment or the
funding status of the sponsor. If this is the case, it is recommended
that instead of discussing the critiques by individual reviewers that you
group your comments in the Introduction by topic. For example: “It
was noted by two of the reviewers that the applicant’s grades were less
than optimal during the first two undergraduate years. I have
addressed this concern by stating that I experienced personal issues
during that time period of my education and I have addressed it more
in depth in the Personal Statement of my Biosketch.” Remember, you
only have one page to describe how you addressed all of the reviewers’
comments and it is essential that you condense wherever possible
without sacrificing clarity and detail.
Finally, it is essential that you conclude the Introduction by inform-
ing the reader how you are indicating changes within the grant: “All
modifications to the grant are indicated by a solid black line in the left
hand margin.” Include the indicated markings wherever you have
modified the grant. It is usually not recommended to utilize bold,
underlined, or italicized font to indicate where the modifications occur.
As discussed before, these types of modified fonts are used to provide
stress and bring attention to key points and statements that you are
making (e.g., hypothesis or significance). Further, a large amount
of bolded, italicized, or underlined sections may look messy and
overly busy.
125Now What?—Resubmission

APPENDIX
CHECKLIST OF REQUIRED ITEMS
The following is a checklist of the major application components
required for all Ruth L. Kirschstein Training grants (F30, F31, F31
Diversity, and F32) that must be written by either the applicant or the
sponsor.
APPLICANT’S RESPONSIBILITY
• Research Training Plan
• Introduction (resubmissions only—1 page)
• Specific Aims (1 page)
• Research Strategy (6 pages)
• Significance
• Approach
• Human Subjects (only if human studies proposed)
• Vertebrate Animals (only if animal studies proposed)
• Bibliography and References Cited (no page limitations)
• Resource Sharing Plan (1 page)
• Facilities (1 page)
• Equipment (1 page)
• Institutional Environment and Commitment to Training (2 pages)
• Respective Contributions (1 page)
• Applicant’s Background and Goals for Fellowship Training
(6 pages)
• Doctoral Dissertation and Research Experience
• Training Goals and Objectives
• Activities Planned Under This Award
• Selection of Sponsor and Institute (1 page)
• Responsible Conduct of Research (1 page)
• Cover Letter (1 page)
• List of Referees (3 referees minimum)
• Applicant’s Biosketch (5 pages)
• Descriptive Title (200 characters in length, including spaces and
punctuation)
• Project Summary/Abstract (30 lines of text)
• Project Narrative (23 sentences in layman’s terms)
SPONSOR/COSPONSOR RESPONSIBILITY
• Sponsor and Cosponsor Information (6 pages)
• Research Support Available
• Sponsor’s/Cosponsor’s Previous Fellows/Trainees
• Training Plan, Environment, Research Facilities
• Number of Fellows/Trainees During the Fellowship
• Applicant’s Qualifications and Potential for a Research Career
• Sponsor’s Biosketch (5 pages)
• Cosponsor’s Biosketch (if required—5 pages)
128 Appendix

ACKNOWLEDGMENTS
I am greatly indebted to the National Institutes of Health publication
Always There: The Remarkable Life of Ruth Lillian Kirschstein, MD
by Alison F. Davis, PhD. This outstanding book served as the primary
source for the brief biography of Ruth L. Kirschstein provided in
Chapter 1, Ruth L. Kirschstein—The Woman and Her Legacy.
